Manufacturing Active Pharmaceutical Ingredients (Api)
Find innovative production technology for making active pharmaceutical ingredients (api) and connect directly with world-leading specialists
API making equipment must observe rigorous quality control standards across the entire manufacturing process. The pharmacodynamic properties of active pharmaceutical ingredients API make them highly sensitive to their environment, making API manufacturing and production a tightly regulated field.
Top technology picks for making API

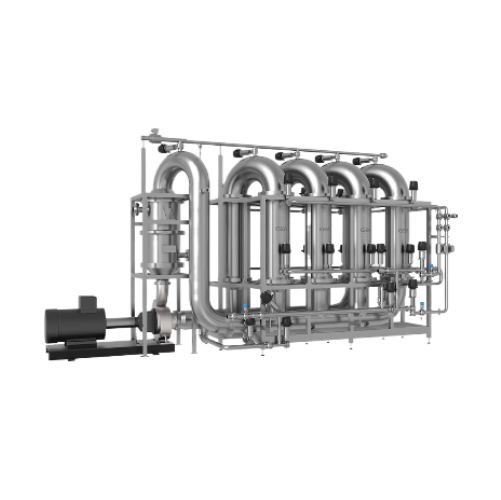
Agitator bead mill for API
To achieve precisely defined API properties and safe and reproducible production, rigorous implem...

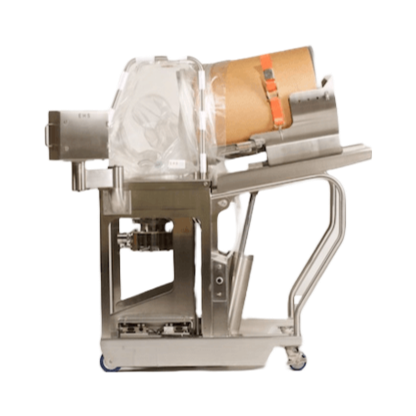
Continuous wet granulator and dryer for R&D
Pharmaceutical laboratories need compact equipment to handle and produce small...

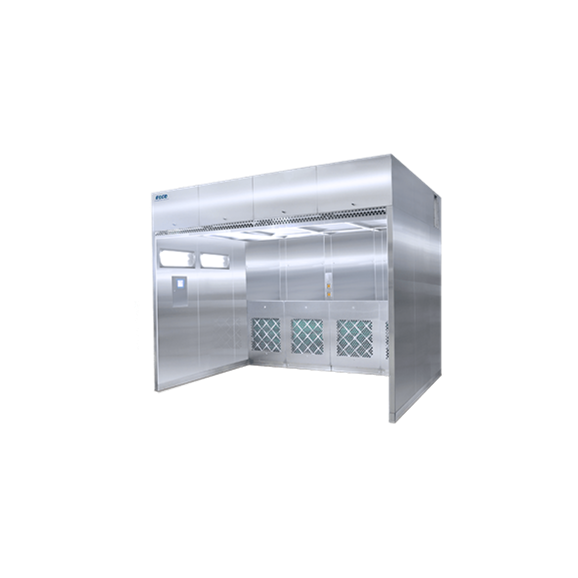
Automatic tablet coater for lab scale
The ability to coat tablets in an even and controlled way is an important stage in th...

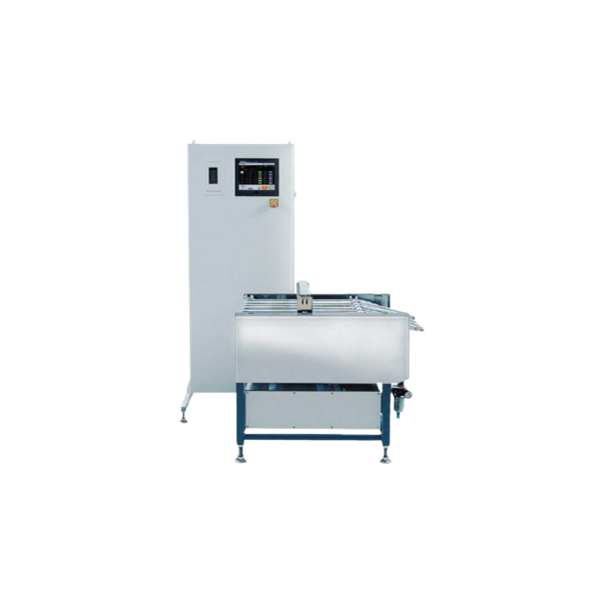
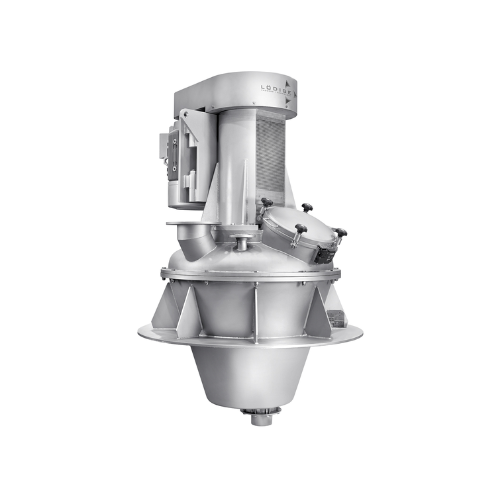
3D powder mixer for active pharmaceutical ingredients
Proper mixing and homogenization are essential to achieve a reliabl...
Stories about active pharmaceutical ingredients (api)

Scientifically tested continuous drying for your tablets

Aim your safety standards high, while granulating dry

Discover the best mix for your blending application

Wet milling: The revolution in the API production

Closing the containment loop in HPAPI manufacturing

Lowering heat stresses on API by reducing friction
Select your active pharmaceutical ingredients (api) process
Tell us about your production challenge
Manufacturing API from a natural, synthetic, or semi-synthetic base
Synthetic APIs are developed from chemical conversions in a lab. Natural APIs, on the other hand, are derived from plants or animals and then purified. Semi-synthetic options stand somewhere in between. They are extracted from natural sources but then biochemically converted into the final Active Pharmaceutical Ingredient API.
The three main groupings of active pharmaceutical ingredients, however, employ similar extraction methods. The intermediaries are extracted from raw materials in a reactor before passing through a series of filtering and centrifuges for isolation and purification. Depending on the finished products, API are milled or freeze-dried before packaging. Distillation is also a key process used in the manufacturing of APIs. The distillation process is used to purify and isolate the desired compound from a complex mixture of chemicals.
Follow GMP standards for your API making equipment
Your API processing equipment must have official certification for reaching production standards.
There are multiple protocols, but Good Manufacturing Practices (GMP) are the global benchmark in pharmaceutical product certification. The World Health Organization (WHO) issues guidelines in this department but other jurisdictions such as China, the EU, and Canada have developed their own GMP.

Classification of API must be clearly displayed on your product label
There are hundreds of active pharmaceutical ingredients used for a wide range of finished pharmaceutical products. Orphenadrine, for example, is the main API found in muscle relaxants, while antivirals are composed of oseltamivir carboxylate. The pharmacological effects of antidepressants are driven by active ingredients such as memantine, amitriptyline, and citalopram. APIs come in various forms such as powders, liquids, tablets and capsules.
The WHO keeps and updates a catalog of all API, ensuring common application and labeling across all pharmaceutical manufacturing markets. The names of these ingredients are non-proprietary and must be displayed under the brand names of packaged pharmaceutical products.

HPAPI manufacturing requires expert containment solutions
The API sector is moving in the direction of highly potent active pharmaceutical ingredients (HPAPI). These special components deliver a stronger drug efficacy than regular API and at a reduced dose. Medicines made from HPAPI minimize side effects and are an important development in the treatment of chronic conditions.
Manufacturing HPAPI, however, raises a series of considerations. Producers must ensure strict handling and cleansing procedures to adhere to occupational exposure limits (OEL) and rigorous quality control to keep their workers safe.
Processing steps involved in active pharmaceutical ingredients (API) making
Which active pharmaceutical ingredients (api) technology do you need?

Separators for pharma extraction
Optimize your pharmaceutical extraction process with innovative centrifugal separation tech...

Pharma extraction clarifier
Achieve superior purity in pharma extraction with efficient separation of solids and liquids, se...

batch centrifuge for solid-liquid separation
Achieve precise solid-liquid separation in high-demand environments with a ma...

Vertical batch centrifuge for solid-liquid separation
Optimize your solid-liquid separation process with high-speed centr...

Horizontal batch centrifuge for chemical industry
Optimize your solid-liquid separation with a centrifuge designed for pr...

Planetary ball mills for fine grinding of materials
Optimize particle size and surface area in your samples with high-ene...

Ultra centrifugal mill for size reduction of soft and medium-hard materials
Achieve precise particle size reduction an...

Drum mills for fine grinding of large volumes
Achieve consistent and precise pulverization of large sample volumes with dr...

High energy ball mill for ultrafine grinding
Achieve nanoscale precision and unmatched particle uniformity with high-energ...

Vibratory disc mill for sample homogenization to analytical fineness
Achieve exceptional precision in sample preparatio...

Ultrafine grinding solution for nanoparticles
Achieve ultrafine particle sizes with high-speed precision, ideal for labora...

Rotating sample divider for large bulk materials
Achieve precise, dust-free division and volume reduction of bulk material...

Fluid bed dryer for bulk material laboratory drying
Efficiently dry and mix organic and inorganic materials with precise ...

Sample dividing solutions for laboratory applications
Streamline your lab’s precision in sample preparation with so...

Industrial powder security screening and de-agglomeration
Ensure product purity by efficiently screening and de-agglomer...

Conical milling for high-efficiency particle sizing
Maximize efficiency in your production line with conical milling tech...

Production-scale milling and screening system
Achieve precise particle size distribution and high throughput rates with a ...

Cone mill for size reduction
Achieve precise particle size reduction and deagglomeration with minimal heat generation, ensur...

Microsphere refiner for aseptic drug manufacturing
Achieve precise control over microsphere drug formulations with this m...

Agitated nutsche filter dryers for chemical synthesis
Enhance your production efficiency by integrating a system that com...

Vacuum tray dryer for pharmaceutical applications
Optimize drying of heat-sensitive compounds while maintaining product i...

Laboratory nutsche filter dryer for solid-liquid separation
Optimize your lab processes with a versatile benchtop filter...

Pilot scale nutsche filter dryer for solid-liquid separation
Achieve maximum product recovery and consistent quality wit...

Nutsche filter dryer system for small scale batches
Efficiently tackle filtration and drying challenges with a compact, m...

Vacuum cold trap for efficient purging and drying
Optimize your laboratory’s efficiency by integrating high-perform...

Diaphragm vacuum pumps for laboratory applications
Optimize your lab processes with oil-free diaphragm vacuum pumps, ensu...

Compact laboratory chiller for recirculating temperature-controlled fluid
Ensure consistent cooling and significant wa...

Immersion chiller for laboratory applications
Achieve precise temperature control in extraction labs and research environm...

Deep water bucket bath for lab heating applications
Achieve precise temperature control and uniform heating for delicate ...

Dissolution profiling system for biorelevant media volumes
Optimize your lab’s efficiency by integrating this dissolutio...

In vitro dissolution and flux measurement platform
Optimize drug formulation by accurately assessing dissolution, solubil...

In-situ fiber optic Uv-vis spectrometer for dissolution studies
Optimize drug formulation by obtaining real-time, in-sit...

Automated platform for determination of physicochemical properties
Optimize your compound screening process with a full...

Dissolution-absorption study system for drug formulations
Optimize drug formulations with precise in-situ dissolved subs...

In-vitro dissolution-absorption assessment
Optimize drug formulation tests by combining dissolution and absorption measure...

Uv monitoring system for small volume dissolution studies
Efficiently monitor small-volume dissolution processes in real...

Low-volume dissolution-absorption testing system
Optimize your development of oral formulations with a device that enables...

15l vertical-wheel bioreactor for cell therapy scale-up
Accelerate your cell therapy scale-up with a bioreactor that offe...

Large-scale bioreactor for cell therapy manufacturing
Tackle the challenge of scaling therapeutic cell production with a ...

High-speed capsule filling system
Maximize production efficiency with high-speed capsule filling designed for precise dosin...

Aseptic bag-in-box filler for laboratory use
Achieve aseptic filling precision on a lab-scale with a compact solution that...
Micro-dosing system for pharmaceutical powders in vials
Ensure accurate aseptic dosing of sensitive pharmaceutical powder...
Lab powder dispenser for precision measurements
Effortlessly streamline your lab’s powder dispensing tasks with a ve...

Laser diffraction particle size analyzer
Achieve precise particle size analysis with laser diffraction technology, ensuring...

Nanoparticle size analyzer
Master precise nanoparticle and zeta potential analysis with dynamic light scattering technology,...
Dished end for optimized mixing in small-volume vessels
When space constraints challenge vessel design, this solution eli...

High power mixing solution for powder applications
Achieve rapid, uniform mixing of challenging powders and non-shear sen...

Flexible powder recipe formulation system
Streamline your batch production with efficient, dust-tight formulation and blen...

Ibc blender for industrial powder mixing
Streamline your powder mixing with rapid batch changeovers and reduced cleaning do...
Wet mixer for viscous and paste-like products
Achieve high-quality mixing of medium to highly viscous products with precis...

Vacuum paddle dryer for laboratory use
Achieve precise temperature and moisture control in your batches with this vacuum sh...

Water activity headspace analyzer for drug product samples
Ensure precise control of moisture levels across the entire p...

Highly scalable reactors for chemical synthesis
Optimize your production line with versatile reactors designed for seamles...
Laboratory mill for dry sample preparation
Achieve precise particle size reduction for pharmaceuticals and fine chemicals ...

Laboratory dispersion reactor for 2l mixing and reaction processes
Streamline your laboratory processes with a versatil...

Super high shear inline disperser for nanoparticle production
Achieve unmatched particle size reduction with cutting-edg...

Powder induction system for high solid concentrations
Optimize your production line with this advanced system designed to...

Laboratory dispersion reactor for 10l batch processes
Enhance precision and efficiency in lab-scale liquid formulations w...

Ultrasonic liquid processor for feasibility testing and procedure optimization
Optimize your liquid formulations by en...

Vibrating control screeners for high volume screening and sifting
Ensure precise material separation with a solution de...

Heavy duty lump breaker for coarse and pre-grinding
Achieve consistent particle size reduction with this heavy-duty solut...

Universal mill for fine grinding in food and pharma industries
Achieve unparalleled particle size reduction with high-pe...

Air classifier mill for ultra-fine powder grinding
Optimize your milling operations with precision particle size control ...

High-speed centrifugal sifter for food and pharma applications
Optimize your powder processing with a high-speed centrif...

Stand dispersing unit for pilot plant applications
Achieve precise particle size reduction and efficient mixing with this...

In-line high shear mixing solution
Achieve precise emulsification and particle size reduction with high shear capabilities,...

Single-pot granulation processor for pharmaceutical applications
For manufacturers needing precise granulation and dryin...

Powder filling and stoppering for antibiotic production
Tackle antibiotic resistance with precision powder filling and st...

Industrial tablet coating solution
Achieve precise film and sugar coatings on a versatile range of tablet and pellet forms,...

Tablet press for high containment environments
For handling potent compounds without compromising safety, a high-containme...

Pilot freeze dryer for pharmaceutical applications
Optimally bridge laboratory development and full-scale production with...

Industrial GMP freeze dryer
Optimize the lyophilization of sensitive pharmaceutical compounds with a machine designed for pr...

Small-scale freeze dryer for pharmaceutical applications
Optimize small-batch and pilot-scale lyophilization with advance...

Continuous aseptic spray-freeze-drying system
Achieve higher sterility and improved product uniformity with a continuous a...

Pharmaceutical components washing station
Ensure optimal cleanliness with high-efficiency washing and drying of pharmaceut...

Customised tank systems for liquid and bulk material storage
Ensure safe and compliant storage of liquids and bulk mater...

Continuous mixing for fine and cohesive powders
Achieve unparalleled mixing precision for cohesive powders while reducing ...

Batch mixer for segregative, free-flowing powders and pastes
Achieve precise and gentle batch mixing for delicate produc...

Powder mixing systems
Optimize your production line with precision powder mixing systems that ensure uniformity, enhance prod...

Choppers and disintegrators for industrial size reduction
Enhance your production efficiency by mastering size reduction...

Compactors and granulators for powdery products
Transform loose powders into dense, free-flowing granules that enhance han...

Drying systems for powders and bulk solids
Enhance your production line with precise control of moisture content in powder...

Lab-scale powder processing system
Achieve precise control in ultra-small batch processing with a versatile modular system,...

Powder characteristic evaluation
Ensure precise powder analysis and testing in your laboratory to optimize production qualit...

Laboratory vacuum dryer for heat-sensitive materials
Optimize moisture control in heat-sensitive materials with precise a...

Containment solutions for hazardous material processing
Ensure safe and efficient processing of hazardous materials with ...
Conical screw mixer for powder blending
Achieve precise and homogeneous blending with the conical screw mixer, ensuring uni...

Pharmaceutical freeze dryer
Achieve contamination-free, high-quality pharmaceutical powders through an innovative freeze-dry...

Conical paddle vacuum dryer for powders and granules
Achieve efficient drying of sensitive materials with this versatile ...

Ultra-fine powder flash drying system
Achieve rapid moisture removal and particle refinement with this integrated system, d...

Ultra-fine powder grinding with jet mills
Achieve unparalleled fineness and purity in powder production with cutting-edge ...

Spiral jet mill for superfine powder production
Achieve consistent ultra-fine powder with precise particle size control, c...

Ultrafine classifier for precise particle separation
Gain precise control over particle size with this ultrafine classifi...

Ultrafine air classifier for powder separation
Achieve precise particle separation with technology designed to enhance you...

Laboratory vacuum milling for fine particle size reduction
Streamline your lab and pilot processes with high-speed vacuu...

Vacuum recirculation mill for particle size reduction
Achieve precise particle size reduction and eliminate air entrapmen...

Immersion mill for pail and drum-sized batches
Streamline your production with a versatile solution that efficiently handl...

Compact full product inspection system for pharmaceuticals
Easily detect fill level inaccuracies, closure misalignments,...

Vial inspection system for pharmaceutical products
Ensure the integrity and safety of parenteral drugs with advanced imag...

Syringe and cannula inspection system
Ensure flawless quality in injectable products with advanced inspection technology th...

Vacuum pan dryer for heat-sensitive pasty products
When dealing with highly viscous, heat-sensitive products, efficient m...

Vertical vacuum dryer for heat sensitive products
Achieve optimal drying and mixing for your heat-sensitive and shear-sen...

Containment system for filter dryers
Ensure safe handling and containment of hazardous materials during sampling and discha...

Chemical filter dryer for fine chemical production
When processing chemical intermediates, precise filtration and drying ...

Filter dryer with containment system for hazardous materials
Ensure operator safety and product integrity during hazardo...

Pharmaceutical filter dryer
Achieve precise moisture control and efficient drying of sensitive materials with this advanced ...

Multilayer filter plates for pharmaceutical and chemical applications
Achieve efficient filtration and separation in yo...

Horizontal vacuum paddle dryer for bulk production facilities
Achieve precise moisture control in powders with a versati...

Horizontal vacuum paddle dryer for sensitive pharmaceutical ingredients
Achieve precise drying and mixing of sensitive ...

Pilot plant filter dryer for small scale production
Optimize filtration and drying processes in your pilot plant with a v...

Pilot unit filter dryer for laboratory applications
Efficiently manage filtration and drying processes with precise tempe...

Sterilizable filter dryer for pharmaceutical applications
Ensure full sterility and efficient moisture removal in critic...

Vertical peeler centrifuge for pharmaceutical industry
Ensure thorough separation and purification of products with this ...

Vertical pilot plant centrifuge for chemical industry
Optimize your lab and pilot plant operations with this mobile centr...

Vertical pilot plant centrifuge for pharmaceutical applications
Ensure precise separation and optimal scaling in your la...

Conical screw cooker for food production
Optimize batch and continuous operations in your production line with efficient mi...

Conical screw vacuum dryer for hazardous and temperature-sensitive products
When you need precise moisture control for...

Vacuum drying solution for filter cake or viscous materials
When faced with the challenge of drying filter cake or trans...

Cylindro conical mixer for batch mixing
Maximize mixing efficiency in compact spaces with this versatile solution, ideal fo...

Laboratory system for drying and mixing processes
Enhance your R&D capabilities with precise drying and mixing contr...

Pilot plant conical screw dryer
Optimize drying efficiency with this versatile pilot plant solution, ideal for simulating an...

Horizontal pilot plant centrifuge for chemical processing
Enhance your R&D and pilot-scale processes with a centrif...

Horizontal pilot centrifuge for pharmaceutical applications
Achieve precise separation of solid and liquid phases with c...

Inverting filter centrifuge for difficult-to-filter products
Designed for challenging filtration tasks, this centrifuge ...

Inverting filter centrifuge for high-potent Api products
Achieve optimal separation and reduce residual moisture with pre...

Separation of cutting fluids from metal chips
Enhance your production line efficiency by effectively separating cutting fl...

Top discharge centrifuge for chemical manufacturing
Achieve precise filtration and separation with top discharge centrifu...

Top discharge centrifuge for pharmaceutical production
Optimize your product yield and quality with precise filtration an...

Classifying centrifuge for wet classification of fine particles
Ensure precise particle classification in continuous pro...

Horizontal peeler centrifuge for chemical applications
Achieve high-throughput product separation and purification with p...

Pharmaceutical horizontal peeler centrifuge
Optimize product consistency and purity with precise separation and filtration...

Lab machine for producing portion packs
Efficiently form, fill, seal, and punch portion packs in laboratory settings with c...

Powder dosing for lab and galenics
Ideal for precise dosing needs, this compact lab device streamlines powder filling proce...

Powder flow control system for gravity transfer
Ensure precise dosing and prevent spillage during powder transfers in your...

Laboratory and pilot scale blending module
Efficiently integrate multiple blending functions on a single drive for streaml...

Split butterfly valve for sterile powder transfer
Ensure safe and sterile transfer of powders with unparalleled containme...

Industrial bin blenders for uniform batch production
Optimize your blending process by achieving uniform mixtures with in...

Ibc tumbler for uniform batch mixing
Achieve consistent and homogenous mixing of powders, granules, and solid products with...

Vial filling for pharmaceuticals
Streamline your aseptic processing with vial filling machines that ensure sterile, precise,...

Laboratory filter-reactor for chemical synthesis and extraction
Optimize your chemical processes with a versatile filter...

Lab nutsche filter for small scale filtration
Efficiently manage filtration and drying needs in fine chemical and pharmace...

Centrifugal disc filter for solid-liquid separation
Optimize solid-liquid separation in your processing line with an adva...

Nutsche filter dryer for pharmaceutical and chemical industries
Achieve precise filtration and efficient drying in one s...

Chemical and pharmaceutical solid-liquid separation pilot plant
Optimize your pilot-scale production of pharmaceuticals ...

Rotary vacuum paddle dryer for pharmaceutical ingredients
Ensure precise moisture control and efficient drying for sensi...

Vacuum paddle dryer for active pharmaceutical ingredients
For precise moisture control and contamination-free conditions...

Spherical vacuum dryer for Apis and fine chemicals
Achieve precise moisture control and efficient drying for APIs and fin...

Vertical conical dryer for pharmaceutical ingredients
Optimize drying and mixing processes for high-value substances with...

Glass lined columns for chemical plants
Optimize your chemical processing with glass-lined columns, designed to ensure maxi...

Glass-lined mixing systems for pharmaceutical industry
Achieve consistent mixing and thorough agitation for complex formu...

Fluid bed granulation system for food and chemical industries
Achieve efficient granulation and drying of liquids with c...

Asme glass-lined reactors for chemical processing
Optimize your chemical processes with high-pressure, corrosion-resistan...

Sterility testing isolator for pharmaceutical labs
Ensure reliable sterility testing in a controlled sterile environment ...

Cost-effective rigid-wall isolator for aseptic applications
Ensure sterility and safety with a versatile isolation and d...

Reusable containers for sterile and toxic material transfer
Ensure safe and contamination-free transfer of sterile and t...

Bioprocess sensor for accurate measurement
Optimize your bioprocessing with advanced sensors designed to deliver precise m...

Gravimetric feeders for pharmaceutical applications
Ensure precise ingredient metering in continuous pharmaceutical proce...
Planetary mill for nano grinding
Achieve precise nano-scale particle production and uniformity for demanding research enviro...

Commercial scale spray dryer for pharmaceutical materials
Optimize the drying of pharmaceutical and industrial materials...

High shear GMP wet granulator
Achieve precise and homogenous wet granulation of powders with adaptable settings for improved...

Mini High shear granulator for wet granulation
Optimize your powder granulation with precision and flexibility, ensuring c...

High shear wet granulation line
Achieve consistent and uniform granules with energy-efficient wet granulation designed to op...

Benchtop spray dryer for drug discovery
Optimize your formulation development with a compact spray dryer that streamlines t...

Filter washing cabinet for industrial cartridge filters
Achieve hygienic standards with precision washing and drying of i...

Roller compaction system for dry granulation
Achieve precise densification and consistent powder granulation with a system...

Laboratory fluid bed granulator and coater
For researchers and manufacturers requiring precise control in drying, granulat...

Development fluid bed system for feasibility studies
Explore precise granulation and coating capabilities for small batch...
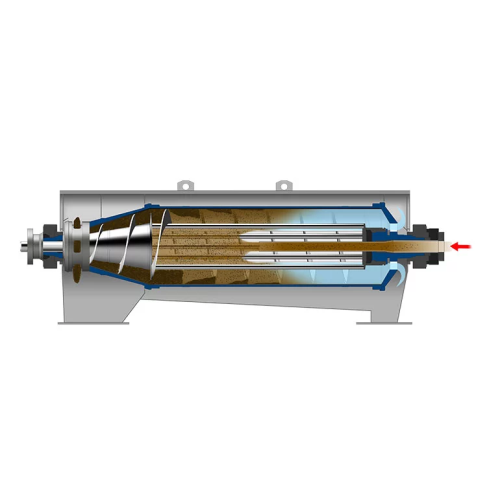
Advanced sludge dewatering for sewage treatment plants
Optimize sludge dewatering with a centrifuge that enhances separat...

Cleanroom air filtration unit for pharmaceutical production
Ensure sterile integrity in your manufacturing process with ...

Pharmaceutical isolator for contamination control
Ensure sterility and safety in pharmaceutical manufacturing with advanc...

Modular cleanroom panel systems
Transform your cleanroom projects with modular panel systems that streamline installation an...

Conical mill for particle size reduction and homogenization
Achieve precise particle size reduction and homogenization w...
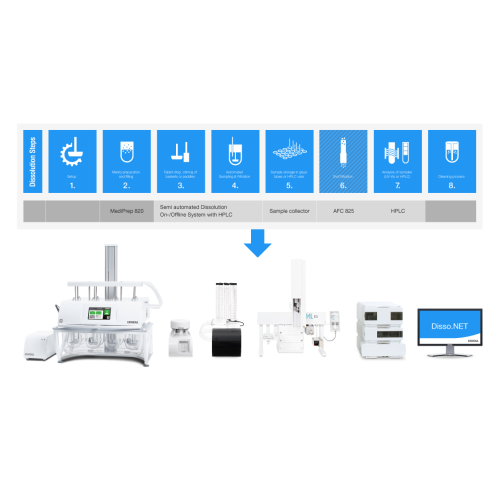
Dissolution testing system with Hplc analysis
Streamline your dissolution testing with integrated HPLC analysis, offering ...

Automatic dissolution testing for sustained release dosage forms
Effortlessly simulate pH changes for accurate dissoluti...

Dissolution online system for automated Uv-vis analysis
Ensure precise dissolution testing and seamless UV/VIS analysis w...

High volume dissolution tester for pharmaceutical research
Achieve precise dissolution testing of tablets across two bat...

Intermittent stick pack machine for pharma and healthcare
Looking to streamline your packaging operations with high-spee...

Central water purification system for laboratories
Ensure consistent supply of ultrapure water essential for critical lab...

Industrial hydrogenation plant for pharmaceutical raw materials
Optimize your hydrogenation processes with a modular pla...

Laboratory mixer and dryer for solids
Enhance your laboratory capabilities with precision mixing and drying for small batch...

Pilot plant for highly viscous applications
Perfect for R&D, this pilot plant efficiently handles complex mixing and ...

Vacuum dryer for free-flowing and pasty solids
Achieve precise drying and mixing with this vacuum dryer, optimizing heat t...

In situ dry-ice snow generator for food and pharma applications
Achieve precise cooling and deburring with this versatil...

Compact laboratory mixer for high-shear granulation
Streamline your R&D processes with advanced mixing and granulati...

High-care big bag filling system for hygienic environments
Ensure precise hygienic filling of Big Bags in high-care envi...

Extraction plant for natural essences and active ingredients
Optimize your extraction processes with a versatile system ...

Crossflow filter for clarified and unclarified wines
Optimize your filtration process with ceramic membrane technology th...

Powder blender for efficient mixing and discharge
Achieve precise mixing and efficient discharge with advanced powder ble...

Glass-lined reactor for enhanced mixing performance
Optimize your chemical processes with a reactor that enhances mixing ...

Pharmaceutical reactor for efficient cleanability
Optimize your production with this reactor, designed for efficient mixi...

Industrial universal reactor for large-scale chemical synthesis
For chemical producers scaling past laboratory setups, t...

Vacuum drying for heat-sensitive products
Ensure moisture-sensitive formulations are dried efficiently without compromisin...

Industrial pan dryer for pharmaceuticals
For heat-sensitive powders and pastes requiring precise moisture levels, this pan ...
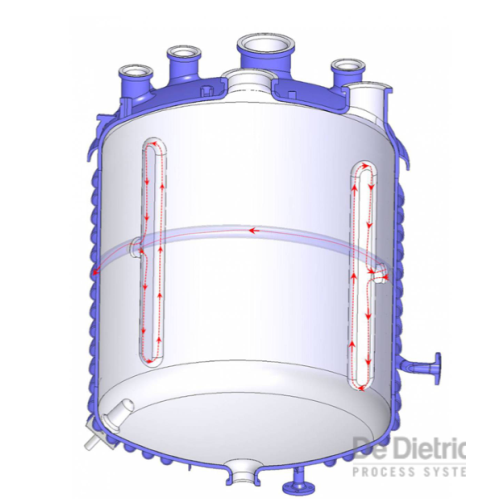
High performance heat transfer system
Enhance your production efficiency with a system that shortens cycle times by increas...

Heat exchanger for corrosive substance handling
Optimize your process of handling corrosive substances with a heat exchang...

Corrosion-resistant shell and tube heat exchangers for pharmaceuticals
Ensure efficient thermal management in high-puri...

Agitated nutsche filter and filter-dryer for pharmaceuticals
Ensure precise filtration, washing, and drying of sensitive...

Glass nutsche filter for fine chemical and pharmaceutical filtration
For precise separation and filtration in R&D ...

Pilot scale filter-dryer for pharmaceutical and chemical batches
Streamline small-batch production with a mobile filter-...

Microwave-enhanced filter dryer for chemical and pharmaceutical applications
Achieve rapid drying and efficient separa...

Static nutsche filters for liquid-solid separation
Achieve efficient separation of solids from liquids with precise contr...

Big bag emptying station for powder handling
Ensure safe and efficient powder discharge with precision containment, adapta...

Mobile powder transfer system for pharmaceuticals and chemicals
Efficiently transfer and charge powders into various ves...

Rotary evaporator for solvent evaporation
Achieve precise solvent recovery and concentration control with this rotary evap...

Thin film evaporator for temperature sensitive products
Need to purify temperature-sensitive compounds efficiently? Achie...

Liquid/liquid extraction mixer-settler plant
Optimize your extraction processes with a highly efficient mixer-settler syst...

12l solid/liquid extractor for pilot plant solutions
Optimize your extraction processes with a versatile extractor that a...
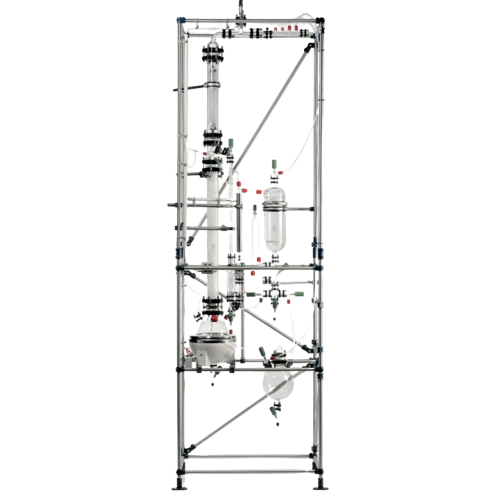
Solid/liquid extraction system for industrial applications
Optimize your solid-liquid extraction needs with a compact mu...

Condensation and separation unit for solvent handling
Enhance solvent management and VOC recovery with this compact conde...
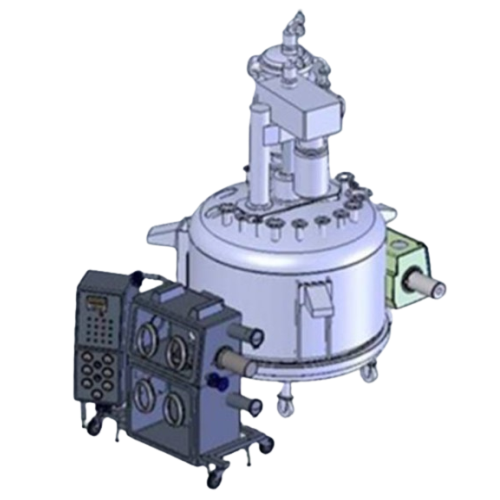
Industrial filter dryer for high potent Api production
Optimize your process of producing high-potency pharmaceuticals by...

Solid/liquid extraction unit for process development
Optimize your extraction processes with flexible operation modes for...

Powder handling drum dock station
Ensure safe and efficient powder transfers with high containment levels, minimizing opera...

Powder conditioning station for high containment applications
Ensure safe and precise handling of hazardous powders with...

Distillation equipment for large reactors
Efficiently achieve high-purity separations and syntheses with distillation equi...
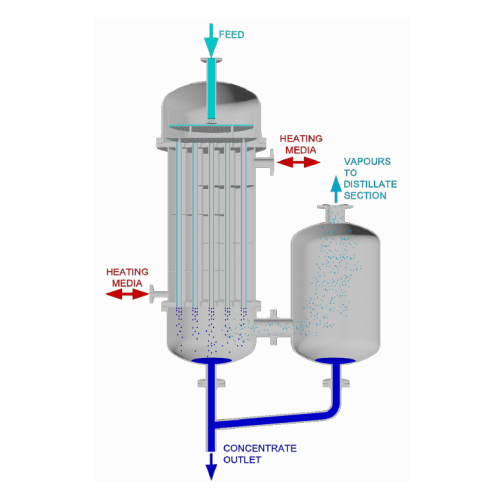
Falling film evaporator for thermal separation
Optimize energy efficiency in separation processes by utilizing gravity-dri...
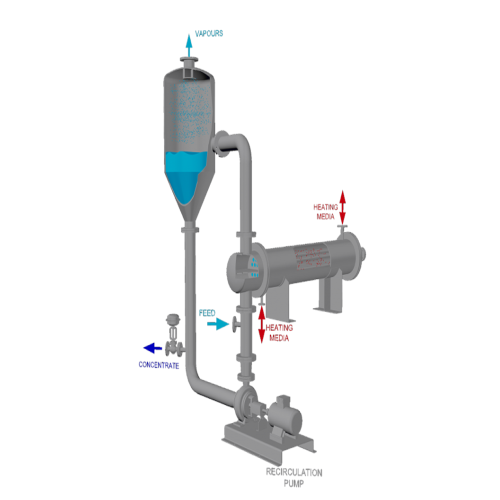
Force circulation evaporator for high viscous products
Optimize energy efficiency and manage challenging liquid compositi...
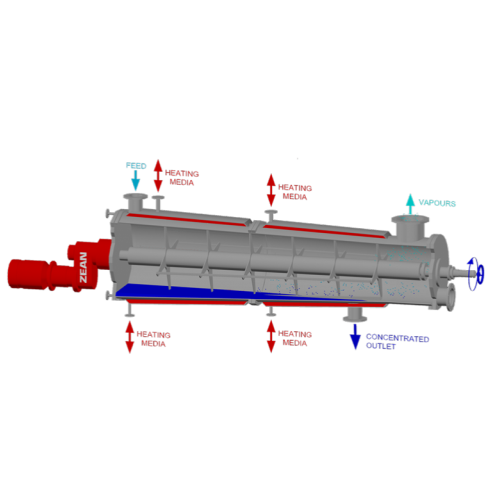
Horizontal thin film evaporator for continuous distillation
Experience precise control over evaporation and distillation...
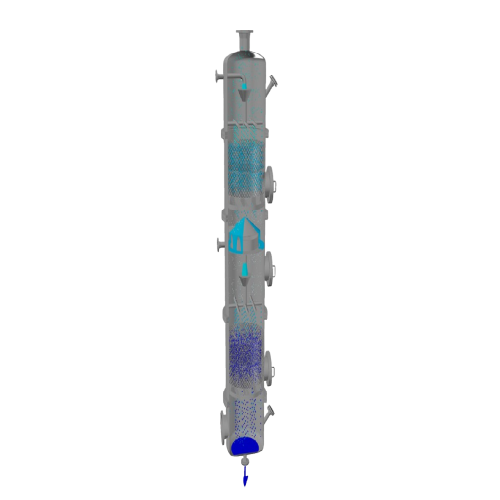
Rectification column for close boiling point separation
Achieve high-purity separation and distillation for compounds wit...

Percolation extractor for high-volume vegetable protein extraction
Optimize extraction efficiency across diverse materi...

General purpose freeze dryer for small commercial batches
Maximize your production line’s flexibility with this ve...

High-volume oilseed extraction system
Achieve optimal extraction efficiencies with a versatile system that handles various ...

Laboratory fluid bed dryer for pharmaceutical granulation
Optimize your lab-scale drying and granulation processes with ...

Wheeled laboratory mixer for powder and granule mixing
Achieve uniform mixing of small powder and granule batches with pr...

Pharmaceutical design feeders for precise ingredient handling
Achieve precise ingredient handling and seamless integrati...

Pharmaceutical twin screw extruders
Optimize the mixing and extrusion of active pharmaceutical ingredients with unmatched p...

Tipping and filling line for bulk Api powder production
Ensure precise and aseptic handling of pharmaceutical powders wit...

Hot cell system for solid target manipulation in radiopharma
Optimize radiopharmaceutical synthesis with precise manipul...

Automatic portable glove tester
Ensure glove integrity in critical environments with rapid, precise leak testing, safeguardi...

Shielded radiochemistry fume hood
Enhance your lab’s safety and efficiency with a fume hood designed for precise hand...

Preparation tank isolator for reactor charging
Ensure aseptic conditions for sterility testing and safe handling of sensit...

Modular dispensing and weighing isolator for pharmaceuticals
Achieve precise dispensing and weighing of high-potency pha...

Mini plant isolator for laboratory protection
Ensure maximum operator safety and product integrity with a versatile isolat...

Shielded cell for radiopharmaceutical synthesis
Ensure operator safety and seamless radiopharmaceutical synthesis with adv...
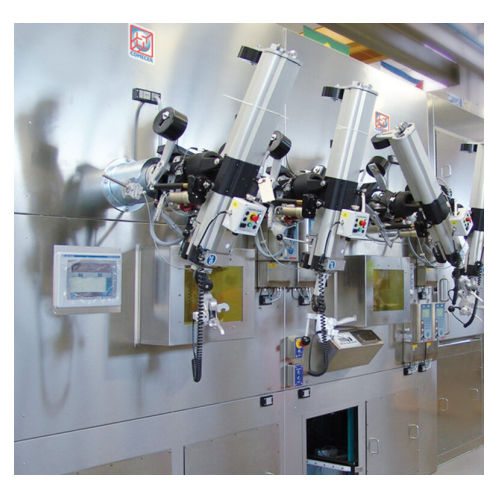
Mo-99 dispensing and packaging hot cell
Safely manage high-activity radiopharmaceuticals with this advanced hot cell, desig...

Shielded isolator for gallium-68 synthesis and dispensing
Optimize your radiopharmaceutical production by ensuring preci...

Isolators for safe Hapi transfer and dispensing
Ensure the safe, precise transfer and dispensing of highly active pharmace...

High containment isolator for wet analysis in pharma
Achieve high containment and precise wet analysis with this isolator...

Laminar flow hood for aseptic preparation
Ensure aseptic conditions for sterile injectable drug preparation with this adva...
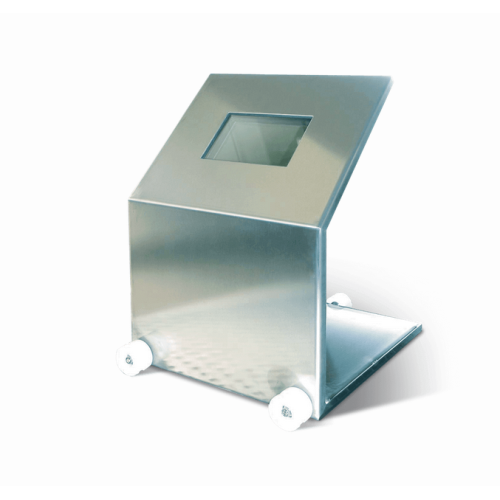
Sliding radiation shield for benches
Enhance safety in laboratory and production environments with a robust bench-top slidi...

Isolator for weighing and dispensing of micronized substances
Ensure safe and precise handling of highly potent substanc...

Atex compliant isolator for dispensing powder procedures
Ensure safe weighing and transfer of explosive and pharmaceutica...

Automated ioflupane i-123 injection dispensing solution
Ensure precision and efficiency in radiopharmaceutical production...
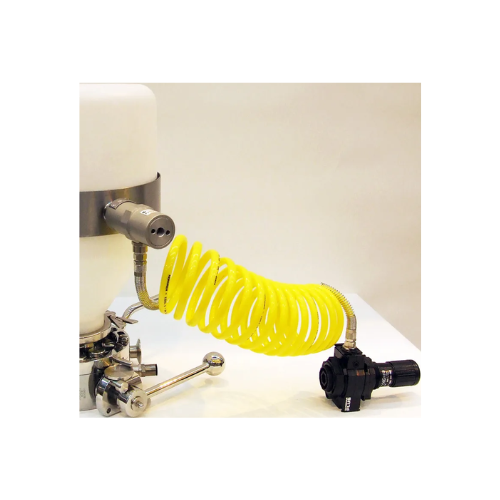
Vibratory collar for powder flow assistance
Struggling with sticky or poorly flowing powders during discharge? This vibrat...

High containment powder transfer valve
Ensure high containment for potent powders in your production line, reducing contami...

High containment powder transfer valve for bulk material
Ensure operator safety and prevent cross-contamination with robu...

Dust particulate extraction system
Ensure safe containment and minimize airborne particulates in your production line with ...

Mobile lifter solutions for pharmaceutical handling
Streamline your handling of sensitive materials with versatile mobile...

Plastic containers for bulk powder transfer
Maximize your facility space with innovative plastic containers designed for e...

Automatic docking system for bulk powder handling
Optimize docking precision and ensure seamless alignment in bulk powder...

Single use valves for powder transfer
Efficiently transfer powder ingredients with a single-use containment solution, ensur...

Transfer valve cleaning for sterility assurance
Ensure seamless cleaning and sterility while maintaining containment integ...

Aseptic transfer valve for sterility assurance
Ensure sterility and safety in your critical production processes with adva...

Autoclave passive opener for sterilisation of passive valves
When sterilising passive valves, ensuring maximum exposure ...

Bottle cleaning station for controlled environments
Ensure high-level decontamination of passive units and containers wit...

Stainless steel container for powder transfer
Ensure seamless and sterile movement of high-value powders with this contain...

Bulk powder transfer safety device
Ensure stable and secure docking by minimizing equipment damage during powder transfer p...

Industrial filter centrifuges for high solids content processing
Optimize processing of high-solid slurries with efficie...

Laboratory oven for accurate thermal processing
Achieve precise thermal control for sensitive formulations, ensuring consi...

High temperature laboratory oven for precise heating
Achieve precise temperature control and uniformity essential for hea...

Split tube furnace for high-temperature applications
Enhance your thermal processing operations with a split tube furnace...

Controlled atmosphere oven for high-temperature applications
Achieve precise atmosphere control and uniform heating for ...

Rotary evaporator for large-scale industrial distillation
Achieve precise separation and concentration with a rotary eva...

Rotary evaporation for laboratory applications
Achieve precise evaporation and concentration with modular, high-speed rota...
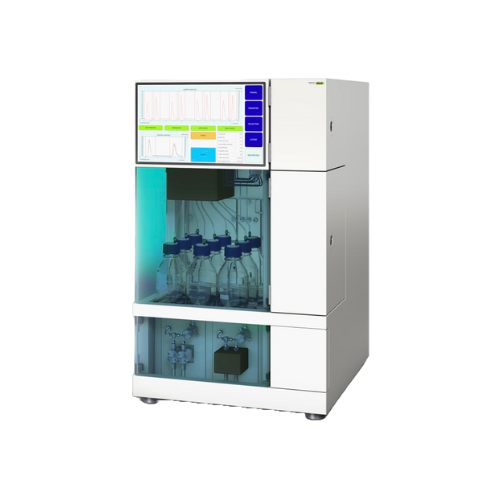
Supercritical fluid chromatography system for preparative separations
For complex substance formulations requiring rapi...

Parallel evaporation system for laboratory sample preparation
Achieve precise sample concentration and drying with autom...

Protein and nitrogen analysis system for laboratories
Streamline your nitrogen and protein analysis with precision titrat...

Nano spray drying system for pharmaceuticals and nanotechnology
Transform minuscule samples into submicron particles eff...

Compact flash and prep Hplc chromatography system
Streamline your purification processes with a versatile system that int...

Flash chromatography system for basic applications
Streamline your purification process with a modular system designed fo...

Industrial recirculating chiller for laboratory applications
For consistent distillation results, maintain accurate temp...

Industrial rotary evaporator for large-scale distillation
Ideal for scaling up laboratory processes, this robust rotary ...

Encapsulation equipment for microbeads and microcapsules
Achieve precise encapsulation of active ingredients and material...

Pilot fermentors for biotech processes
Optimize your pilot-scale production with versatile fermentors and bioreactors desig...

Raw material handling systems for plastic and pharmaceutical industries
Effortlessly manage and optimize the handling a...

Organic solvent nanofiltration for liquid separation
Optimize your process streams with solvent recovery that minimizes e...

Pervaporation membrane for solvent dehydration
Efficiently separate and dehydrate solvents at molecular levels, ensuring p...

Laboratory crossflow filtration test system
Efficiently test and determine optimal filtration parameters with this portabl...

Compact filtration device for laboratory and field use
Achieve precise filtration with minimal sample sizes for rapid ana...

Lab-scale in-situ sterilizable bioreactor
Ensure precision in your laboratory and pilot projects with this bioreactor, des...

Lab-scale fermenters for research and development
Optimize your small-scale production with compact bioreactors designed ...

Industrial homogenizer for food and beverage applications
For manufacturers seeking consistent quality, this homogenizer...

Sterile transfer chamber for pharmaceutical components
Ensure the sterility of your components rapidly with an enclosed c...

Vertical ribbon vacuum dryer for thermosensitive products
Achieve optimal drying of thermo-sensitive materials like powd...

V shape mixer for solids and powders
Ideal for preserving the delicate structures of fragile materials, this mixer ensures ...
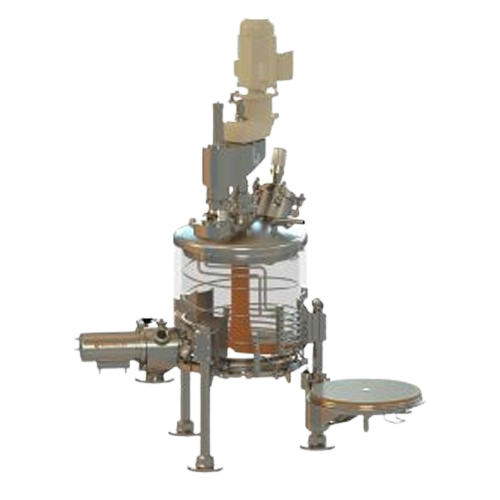
Agitated nutsche filter for solid-liquid separation
Ensure high-purity solid-liquid separation with minimized contaminati...

Agitated nutsche filter dryer for solid-liquid separation
Achieve high-purity solid-liquid separation with optimized thr...

Vertical ribbon mixer for solids and powders
Achieve rapid, uniform mixing of powders and solids while preserving particle...

Horizontal vacuum paddle dryer for thermosensitive products
For high-viscosity products like active pharmaceuticals or p...

Horizontal vacuum paddle dryer for thermosensitive products
Achieve precise drying and mixing of high-viscosity, thermos...

Industrial vacuum mixer and reactor for liquids
For manufacturers needing precise blending and stability, this advanced va...

Vacuum mixer reactor for liquids and semi-solids
Achieve precise mixing and emulsification of complex liquid formulations ...

Conical screw mixer for solids and powders
Achieve precise homogenization of your solid and powder mixtures with minimal e...

Double cone vacuum dryer for thermosensitive products
For precise drying of heat-sensitive powders, this double cone vacu...

Automatic minor components weighing system
Streamline precision in your production by automating the weighing of minor and...

Nutsche filter-dryer for solid-liquid separation
Ensure precise moisture control and efficient solid-liquid separation for...

In-line external homogenizer for industrial fluid processing
Maximize fluid consistency and ensure efficient material di...

Flexible isolator for aseptic containment
Ensure sterility and safety with a flexible isolator designed for efficient cont...
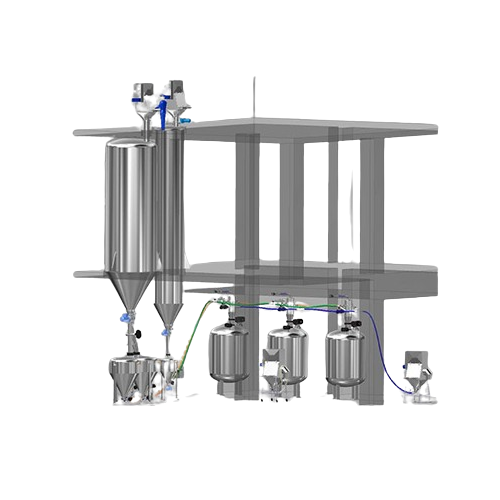
Automatic powder vacuum conveying system for infusion production
Enhance your production line with our vacuum conveying ...

Fine chemical powder system
Optimize your powder processing with a solution tailored to handle complex feeding, conveying, a...

Commercial freeze dryer for pharmaceutical Api production
Ensure the stability and potency of your biopharmaceutical pro...

Microspheres process equipment for classification, washing, drying
Achieve high yield production of polymeric microsphe...

Sterile Api production line for powder treatment
Achieve seamless sterile API production by integrating precise weighing, ...

Non-sterile Api production line for powder and solid formulations
Ensure a dust-free, automated process for pharmaceuti...

High containment isolator for toxic and active drug handling
Ensure safe handling and environmental protection while pro...

Jet mill for micronizing pharmaceutical powders
Achieve precise particle size reduction with a jet mill that leverages hig...

Bead mill for nano-preparation grinding
Achieve precise nano-processing and efficient wet grinding for pharmaceuticals and ...

Particle size control equipment for pharmaceutical applications
Achieve precise particle size control for diverse materi...

Wet granulating equipment for pharmaceutical industry
Enhance production efficiency and safety in high-potency pharmaceut...

Continuous wet granulation equipment
Optimize your production line with continuous wet granulation, seamlessly transforming...

Ultrafiltration and depth filtration system for biopharmaceuticals
Achieve precise separation and purification of bioph...

Pilot-production freeze dryers for diagnostic kits and tissue banking
Optimize your freeze-drying process with compact,...

Customizable production freeze dryer for pharmaceuticals
Ensure precise moisture control and preservation with a versatil...

Aseptic filling line for biopharmaceuticals
Streamline your aseptic filling process with precision dosing and minimal spac...

Pharma peeler centrifuge for high purity separations
Achieve unparalleled product purity and yield in demanding pharmaceu...
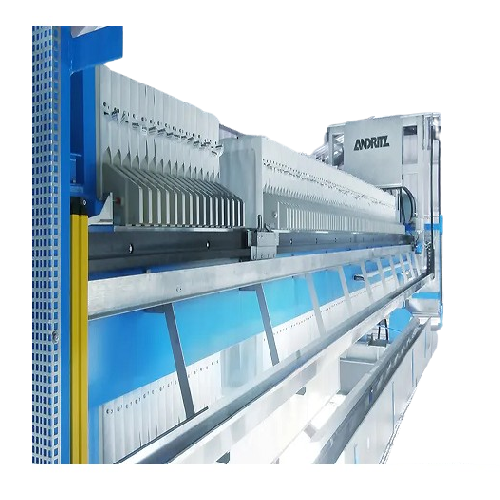
Industrial filter press for dewatering and filtration
Optimize your dewatering and filtration needs with a highly adaptab...

Vertical vacuum dryer and mixing reactor
Achieve precise control over drying and mixing processes with a versatile unit des...

Compact cross-flow membrane filtration system for lab use
Optimize your lab’s filtration process with this compact...

Yeast centrifuge separator for pharmaceutical applications
Optimize microorganism recovery with high purity and efficien...

Mixproof valve for cleaning-in-place control
Streamline your cleaning processes and ensure product safety with a versatile...

Compact industrial boiler for steam and hot water applications
Experience reliable steam and hot water generation with t...

Pharmaceutical roller compactor for precise granulation
Achieve consistent and high-quality granulation with a solution t...

Pharmaceutical roller compactor for dry granulation
When precision and uniformity in tablet production are crucial, optim...

Industrial grater and shredder for pharmaceutical and chemical applications
Optimize your production line with a versa...

Bin blender for pharmaceutical powder mixing
Achieve uniform mixing and homogenization of dry powders directly in storage ...

Lifting column for drums and bins
Optimize your powder handling operations with a versatile lifting solution designed for p...

Mobile bin blender for mixing and homogenizing dry powders
Ensure thorough powder uniformity and eliminate cross-contami...

Powder dosing and weighing system
Optimize precision in pharmaceutical processing with a system designed to ensure accurate...

Mobile lifting column for drum handling
Streamline your powder and drum handling operations with this versatile mobile lift...

Ped-compliant vacuum conveyor for powder transfer
Achieve safe and efficient powder transfer in pressure environments wit...

Vacuum transfer system with integrated cleaning
Ensure sterile powder handling and contamination-free production with vacu...

Hygroscopic material lump breaker
Ensure seamless processing by breaking down compacted or hygroscopic powders efficiently,...

300l stainless steel dual-jacketed reactor for winterization
Enhance your extract processing with precision temperature ...

Pharmaceutical large freeze dryer with vacuum pump
Preserve the integrity and potency of sensitive compounds with precisi...

Pharmaceutical freeze dryer for medium scale production
Ensure optimal preservation of active ingredients through precise...

Nsf certified class Ii a2 biosafety cabinet
Ensure laboratory safety and specimen integrity with a biosafety cabinet desig...

Analytical balance for precise laboratory measurements
Achieve unparalleled precision and reliability in your lab’s...

Ultra-microbalance for precise weight measurement
Achieve unparalleled precision in weight measurement with an ultra-micr...

High precision analytical balance for laboratory use
Ensure precision and reliability in your laboratory measurements wit...

Moisture analyzer for laboratory samples
Accurate moisture analysis is crucial for ensuring product quality in pharmaceutic...

High-precision analytical balance for laboratory use
Achieve precise measurements and ensure reliability in your laborato...

Cold trap for laboratory applications
Achieve efficient condensation and separation in laboratory processes with this versa...

Bridgman crystal growth furnace for advanced crystallization processes
Achieve high precision in crystal growth with th...

20l glass reactor for crystallization and isolation processes
Optimize your lab’s crystallization and isolation pr...

Stainless steel crystallization reactor 100l
Optimize your crystallization and formulation processes with precision-contro...

Decarboxylation package for botanical extraction
Optimize decarboxylation and reaction processes with precise temperature ...

Decarboxylation package for botanical extraction
Ensure precise temperature control and efficient reaction conditions in y...

Dual asymmetric centrifugal processing system for epoxies and slurries
Streamline complex formulations with a system th...

50l jacketed glass reactor with explosion-proof motor
Enhance your reaction processes with precise temperature and pressu...

Laboratory -40°c upright freezer
Ensure optimal preservation of sensitive biological samples with a freezer engineered for u...

Dual-jacketed reactor for decarboxylation processes
Streamline your decarboxylation process with a dual-jacketed reactor ...

Dual-jacketed reactor for botanical extraction winterization
Optimize your production with precise temperature control a...

100l laboratory jacketed glass reactor
Optimize your batch processing with this 100L jacketed glass reactor, designed for p...

10l glass reactor system for synthetic reactions
Achieve consistent reaction control with efficient temperature and vacuum...

20l jacketed glass reactor for controlled chemical reactions
Optimize your chemical synthesis with precise temperature c...

20l jacketed glass reactor system for laboratory synthesis
Achieve precise chemical synthesis and enhanced material reco...

20l jacketed glass reactor
Optimize your chemical reactions with precise temperature control and adaptive stirring capabilit...

Large capacity jacketed glass reactor
Achieve precise control over temperature and vacuum conditions with this versatile re...

1200°c Pe/cvd furnace for high-temperature material synthesis
Enhance your material processing capabilities with precise...

High-temperature tube furnace for laboratory applications
Achieve precise thermal treatment with this high-temperature t...

24-ton laboratory pellet press
Achieve precise pellet formation with reliable 24-ton pressing power, ideal for compaction of...

Controlled atmosphere top-open tube furnace
Achieve precise thermal processing and sintering with this versatile tube furn...

High-shear mixer granulator for pharma and nutraceuticals
Optimize your batch and high-speed production with a powerful ...

High-shear mixer granulator for small batches
Achieving consistent particle size and mix uniformity in pharmaceutical and ...

High-shear granulator for wet granulation
Achieve precise content uniformity with advanced high-shear granulation, designe...

Containment tablet coater for high-potency drugs
Ensure operator safety while achieving precise tablet coating on high-pot...

Tablet coater for batch sizes up to 250 liters
Enhance your tablet production process with high-speed coating solutions de...

Containment tablet coater for high-potency pharmaceuticals
Ensure operator safety and maintain stringent OEB 4 complianc...

Tabletop granulation system for small batches
Enhance R&D capabilities with this innovative solution designed for gra...

Versatile fluid bed combo for granulation and coating
Optimize lab-scale R&D with an adaptable fluid bed system, sea...

Intermediate-scale fluid bed system for drying and granulation
Achieve precise drying and granulation with this versatil...

Advanced fluid bed for pharmaceutical granulation
Achieve precise granulation and drying for pharmaceutical powders with ...

Fluid-bed granulator for top-spray applications
Need precise granulation and drying for sensitive materials? This fluid-be...

Fluid-bed drying and granulation system
Optimize your production line with high-speed batch processing for efficiently dryi...

Integrated granulation train for pharmaceutical processes
Achieve precise granulation, uniform mixing, and efficient dry...

High-shear mixer for large-scale pharmaceutical production
Optimize your granulation process with an advanced high-shear...

High-shear mixer for small batch granulation
Enhance your production efficiency with a high-shear mixer designed for preci...

Containment capsule filler for highly potent drugs
Achieve complete operator safety while encapsulating potent pharmaceut...

Containment capsule filling system for highly potent drugs
Ensure operator safety while filling capsules with high-poten...

Fluid bed dryer with granulation and coating
Enhance your processing efficiency with a versatile system capable of combini...

Fluid bed combo for granulation and coating
Achieve seamless granulation and coating with batch and high-speed operations,...

Fluid bed combo for granulation and coating
Streamline your manufacturing with a versatile fluid bed combo, integrating gr...

Containment blister packaging for highly potent Apis
Ensure operator safety during the blister packaging of highly potent...

Clean-in-place system for diverse processing needs
Ensure the precision and consistency of your cleaning cycles with a ro...

Tablet compression module for high containment applications
Quickly switch between tablet batches without compromising s...

Pharmaceutical direct compression with linear blending
Streamline your pharmaceutical production with a continuous direct...

Containment valves for pharmaceutical powder transfer
Ensure safe transfer of highly potent pharmaceuticals with this mod...

Disposable containment system for solid dosage material
Ensure safe and dust-free transfer of hazardous materials with ou...

Ibc post hoists for pharmaceutical manufacturing
Ensure precise material handling with advanced lifting solutions designed...

Contained powder sampling system
Ensure contamination-free sampling of active powders with fully contained system integratio...

Pharmaceutical intermediate bulk containers (ibc)
Streamline pharmaceutical production with precise containment and trans...

Continuous granulation and drying system for r&d
Develop precise pharmaceutical granules with this compact system, tailore...

Pharmaceutical r&d granulation system
Optimize your pharmaceutical research with a versatile small-scale granulation system...

Pharmaceutical homogenization skid system
Achieve precise particle size reduction and efficient cell rupture with an auton...

High shear granulator for pharmaceutical applications
Efficiently transform powders into uniform granules for consistent ...

Pharmaceutical bottom-drive high shear granulator
Achieve optimal granule uniformity and precise control over moisture le...

Integrated small-scale granulation and drying system
Ensure precise granule size and moisture control for enhanced tablet...

Integrated granulation and drying system for pharmaceuticals
Achieve seamless granulation and drying with our fully inte...

Bubble column reactors for chemical reactions
Enhance gas-liquid interactions with bubble column reactors designed for eff...

Compact plate evaporator for temperature-sensitive products
Ensure optimal heat transfer and minimal space usage with th...
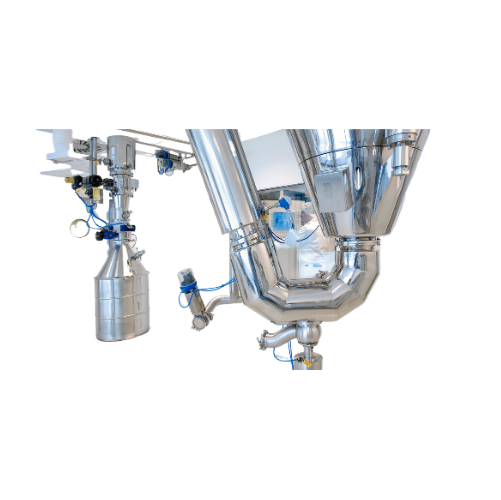
Pharmaceutical spray dryer
Unlock the potential of spray drying to enhance bioavailability, achieve controlled release, and ...

Spray dryers for ceramics and pharma products
Achieve consistent powder quality for heat-sensitive and sticky products wit...

Fluid bed pellet coating solution
Achieve precise and uniform pellet coating with fluid bed technology for enhanced product...

Pharmaceutical fluid bed processor for r&d
Optimize your pharmaceutical R&D with a versatile fluid bed processor desi...
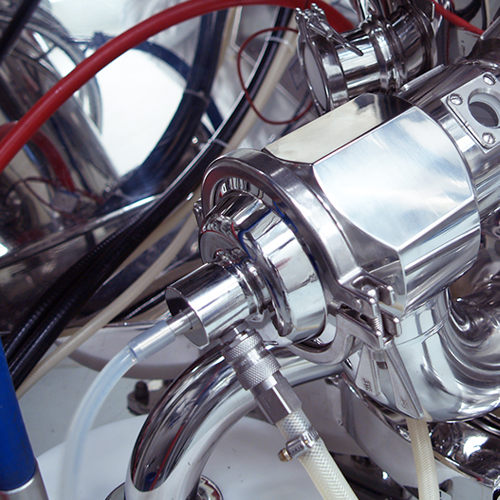
Flexstream fluid bed processor for pharmaceutical applications
Addressing the limitations of traditional fluid bed proce...

Batch distillation plants for diverse compound mixtures
Optimize your separation processes with flexible batch distillati...

Multiple-effect distillation plants for industrial applications
Optimize your energy use with multiple-effect distillati...

Mvr and Tvr heated distillation system
Achieve superior energy efficiency and reduced steam consumption in your distillatio...
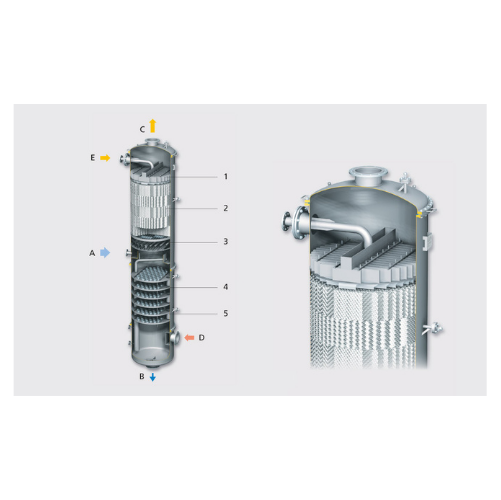
Industrial tray and packing column solutions
For efficient separation and product purification, tray and packing columns o...

Spray dryer for food and dairy products
Achieve consistent drying for diverse powders and granulates with precision technol...

Centrifugal separator for pharmaceutical extraction
Optimize your extraction process with precision centrifuging that enh...

Chemical and mineral processing decanter centrifuge
Achieve efficient clarification and dewatering of complex mixtures in...

Clarifying decanter for chemical processing
Optimize your clarification and dewatering processes with a versatile decanter...

Laboratory vacuum drying system
Achieve precise moisture removal and maintain product integrity with advanced vacuum drying,...

Autoclaves for laboratory and production with square chamber
Optimize your laboratory space with compact autoclaves that...

Combined Avi and Cci tester for vials
Ensure comprehensive quality control of parenteral products with an integrated soluti...

Vacuum conveyors for explosive and inert gas environments
Optimize the safe transfer of hybrid mixtures and fine powders...

Powder locks for safe bulk material handling
Ensure containment and prevent contamination while processing sensitive bulk ...

Pharmaceutical vacuum conveyors for hygienic transport
Ensure contamination-free transport of sensitive pharmaceuticals a...
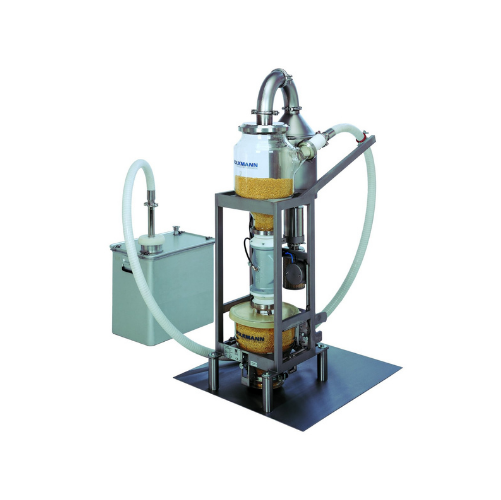
Vacuum conveyor for tablets and capsules
Ensure the integrity of your tablets and capsules with gentle vacuum conveying des...

Big-bag unloading station for bulk materials
Facilitate seamless integration into your production line with efficient bulk...

Drum discharge system for bulk material handling
Achieve precise, gentle transfer of sensitive pharmaceuticals and granula...

Explosion-proof production disperser for paint manufacturing
Ensure safe and efficient dispersion in volatile environmen...

Explosion-proof bead mill for industrial grinding applications
For manufacturers seeking precise particle size control, ...

Explosion-proof horizontal bead mill
For manufacturers seeking precise particle sizing, this explosion-proof bead mill ensu...

Horizontal bead mill for ultrafine particle grinding
Achieve precision in ultrafine grinding with a system that allows fo...

High-capacity dissolvers for industrial mixing
Enhance your production line with precision mixing, achieving consistent di...

High shear batch process homogenizer for production
Achieve efficient emulsifying, homogenizing, and dispersing with prec...

Dissolving system for high-viscosity products
Optimize the dispersion of high-viscosity liquids and pastes with this versa...

Explosion-proof dissolver for high-viscosity products
Optimized for safely processing high-viscosity products, this explo...

Explosion-proof vacuum dissolver for high-viscosity products
Optimize your production by eliminating air inclusions and ...

Basket mill with integrated dissolver for high viscosity products
Optimize your production efficiency by combining disp...

Explosion-proof horizontal bead mill for fine grinding
Ensure precise, explosion-proof grinding in hazardous environments...

Atex horizontal bead mill
Ensure precision and safety in explosive environments with a robust solution that finely grinds an...

Explosion-proof dissolver for hazardous area mixing
When operating in hazardous environments, maintaining consistent and ...

Lab stirrer for high-viscosity substances
Need precise, high-torque stirring for your complex formulations? This lab stirr...

Laboratory and pilot plant horizontal bead mill
Achieve precision milling with minimal product waste, ensuring consistent ...

Rotary homogenizer for laboratory and pilot plant
For achieving optimal dispersion in complex formulations, this solution...

Dissolver for mixing and processing in lab and pilot plant
Optimize your material dispersion and homogenization processe...

Pressure monitoring solution for dosing processes
Optimize your production line by ensuring precise pressure monitoring, ...

Side-mount agitator for large tank mixing
Achieve efficient mixing in large tanks with reduced energy consumption by lever...

Industrial stativ mixer with adjustable height
Achieve precise mixing and stirring across diverse batches with a mobile, a...

Tank agitator for low-level installation
Efficiently optimize your mixing processes with a bottom-entry tank agitator, desi...

Laboratory dedusting systems
Designed for high-containment lab environments, this solution efficiently captures and manages ...

Containment single-use filter module
Ensure safe handling of high-potency substances and streamline filtration processes wi...

Compact dust extractors for cleanroom applications
Ensure safe and efficient dust extraction in cleanroom environments wi...

Industrial particle size reduction processor
Optimize your production line with high-capacity, continuous particle size re...

Handheld particle counters for cleanroom environment monitoring
Ensure precision in contamination monitoring and quality...

Gentle rocker for dissolving active drug in Ngi tray
Experience consistent and reproducible sample preparation with a roc...

Robotic sterilization logistics system
Ensure seamless aseptic transfers across production stages with this robotic logisti...

Pharmaceutical autoclave for terminal sterilization
Optimize your sterile production with high-precision terminal sterili...

Pharmaceutical isolator system for aseptic production
Ensure aseptic conditions and operator safety with a robust isolati...

Feeding isolator for liquid preparation in pharmaceuticals
Ensure precise and contamination-free preparation of liquid p...

Active pharmaceutical ingredient isolation system
Ensure the aseptic production of pharmaceuticals while protecting perso...

Sterility testing isolator for aseptic environment
Ensure precise sterility testing and prevent contamination with an iso...

Automatic chromatography column for biopharmaceutical separation
Ensure high-purity monoclonal antibodies and herbal ext...

Complex preparation system for industrial microsphere production
Achieve precise particle size and uniform drug encapsul...

Industrial freeze dryer for pharma
Optimize moisture removal in lyophilized products with precise thermal conduction and ef...

Twin-screw extruder for high containment applications
Achieve precise mixing of sensitive compounds with a twin-screw ext...

Mini extruder for early development phase testing
In early development, tackling limited material availability is crucial...

Flat-bottom twin-screw feeder for precise powder feeding
Achieve precise control in powder dispensing with this flat-bott...

Precise volumetric or gravimetric powder feeder
For precise feeding of challenging powders prone to clumping and bridging,...

Isolator high containment for granule production
Ensure high-purity production in pharmaceuticals with controlled granulat...

Benchtop x-ray diffractometer for routine analysis
Achieve high-precision phase analysis and crystalline characterization...

Twin-screw extruder for pharmaceutical formulations
Streamline your pharmaceutical production with a dual-purpose extrude...

Micronization for pharmaceuticals
Achieve ultra-fine particle size reduction for complex materials, essential when precisio...

Jet milling system for super fine particle size reduction
Achieve ultra-fine particle sizes with precision milling techn...

Atex approved hammer mills for high-capacity particle size reduction
Ensure precise and reliable particle size reductio...

High accuracy hammer milling for precise particle size reduction
Achieve precise particle size control to enhance produc...

Lab scale hammer mills for fine particle size reduction
Optimize your R&D scaling efforts with versatile lab equipme...

Vacuum extruder for high-viscosity materials
Optimize material processing with precision de-airing and temperature control...

High-pressure hydrogen generator for laboratory use
Replace cumbersome hydrogen cylinders with an on-demand generation so...

High-temperature and high-pressure flow reactor
Achieve unparalleled precision in high-temperature and high-pressure chemi...

Remote control software for laboratory flow reactors
Streamline your laboratory experiments with a system that provides s...

Automated liquid handler for hydrogenation reactions
Enhance reaction efficiency and precision in laboratory settings wit...

Continuous flow hydrogenation reactor for corrosive reagents
Transform your hydrogenation processes with a reactor engin...

Catalyst cartridge switching system for flow chemistry
Effortlessly switch between multiple catalysts in a single run, op...

Catalyst cartridge packing system
Efficiently pack and seal catalysts into cartridges with precision, ensuring reliable and...

Reusable catalyst cartridges for safe hydrogenation
Simplify catalyst handling and enhance reaction control with reusable...

Hydrogenation reactors for safe and efficient syntheses
Experience safe, on-demand hydrogen generation for precise flow h...

Advanced hydrogenation in continuous flow
Achieve precise, on-demand hydrogenation with advanced safety features, eliminat...

Automatic mono-dose strip filling system
Optimize your liquid product packaging with a multi-functional system designed for...

Saturated steam sterilizer for pharmaceutical production
Ensure sterility for a diverse range of pharmaceutical products ...

pharmaceutical lyophilised filling line
For high-precision production of moisture-sensitive drugs, this aseptic system ensu...
Sublimation front monitoring system for freeze drying
Ensure precise control over your freeze-drying process with real-ti...

Sterile lyophilised filler
Achieve precise weighing and contamination-free lyophilisation with our cutting-edge sterile fill...

Sterile lyophilized filling line
Ensure sterility and precision in filling pharmaceuticals with advanced isolator lines desi...

Sterile lyophilised filling solution
Achieve precise filling and control of moisture-sensitive pharmaceuticals while ensuri...

Two lyophiliser unloading isolators for hygroscopic products
Ensure precise moisture control and sterility for hygroscop...

Chest ultra low temperature freezer for biological sample storage
Efficiently preserve high-value biological and medica...

Containment isolators for pharmaceutical and biotech applications
Ensure operator safety and product integrity during t...

Downflow booths for hazardous material processing
Ensure product integrity and operator safety during hazardous material ...

Sterility test isolator for pharmaceutical processes
Ensure precise sterility testing with an isolator that maintains an ...

Basic research freeze dryer for biomedical applications
For laboratories seeking precise sample preservation, this bencht...
Continuous manufacturing for pharmaceuticals
Achieve precision in oral solid dosage production with seamless continuous pr...

Pharmaceutical aseptic separator filter dryer
Achieve seamless filtration, washing, and vacuum drying in a single aseptic ...

Lab Pharmaceutical aseptic separator
Achieve high-yield recovery and safe handling of potent pharmaceuticals with this asep...

Pilot pharmaceutical aseptic separator and dryer
Achieve high-yield recovery of potent compounds with a separator that com...

Pharmaceutical aseptic separation system
Achieve precise separation and efficient drying of pharmaceutical powders with an ...

Pharmaceutical aseptic separator tumble dryer
Streamline moisture reduction with precision: this solution accelerates dryi...

3d metal printer powder screening system
Enhance your 3D metal printing workflow by efficiently reclaiming and rebottling u...

Sterilization system for biosafety applications
Ensure sterility and compliance in critical manufacturing processes with a...

Mobile biodecontamination unit for small to mid-size rooms
Efficiently achieve 6-log bioburden reduction in controlled e...

Pharmaceutical grade washer for large components
Ensure compliance and maximize productivity with a high-performance solut...

Vacuum rated vibro sifter for pharmaceutical materials
Ensure precise separation of impurities and achieve consistent par...

Medium torque top entering mixer for high fluid forces
Equip your production line with a robust mixer that transforms cha...

Tall form dryer for producing large non-agglomerated particles
Achieve precise particle size control and optimal moistur...
Pharmaceutical sieving equipment for high hygiene standards
Ensure the purity of pharmaceutical powders and granulates w...

Pilot-production freeze dryer for diagnostic kits and Api
Optimize your lyophilization process with this versatile freez...

Production freeze dryer for pharmaceutical manufacturing
Achieve precise lyophilization for sensitive products with custo...

Refrigerated vacuum cold trap for laboratory vapors
Protect your vacuum pumps by efficiently trapping harmful condensable...

Semi-automatic tablet hardness tester
Optimize your tablet production with precise and reliable testing of hardness, dimens...

Tapped density tester for pharmaceutical powders
Ensure precise quality control in pharmaceuticals by accurately measuring...

Automated weighing system for tablets
Ensure precision in pharmaceutical manufacturing by seamlessly integrating advanced w...

Automated sample preparation workstation for lab efficiency
Streamline laboratory operations by automating sample prepar...

Automated sample preparation workstation for content uniformity testing
Streamline your laboratory workflows with autom...
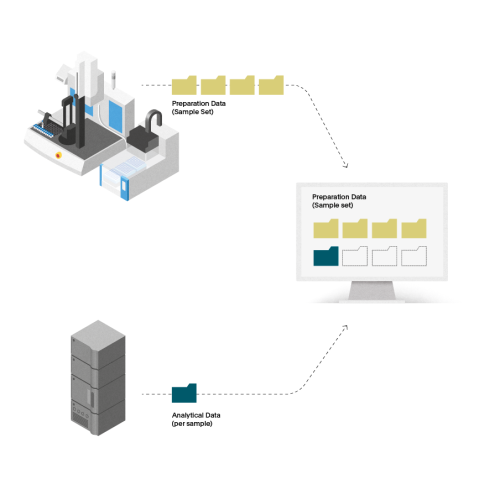
Automated sample preparation software for pharmaceutical testing
Streamline your laboratory’s sample preparation w...

Dissolution medium preparation system
Streamline your laboratory workflows with a system that prepares and manages dissolut...

Automated flow-through cell dissolution testing system
Enhance precision in dissolution testing with real-time UV-Vis ana...

Flow-through cell dissolution testing with Uv analysis
Effortlessly monitor real-time dissolution with flow-through cell ...

Manual tablet disintegration tester
Ensure precise and reliable disintegration testing of pharmaceutical tablets, capsules,...

Tablet friability tester
Ensure your tablets meet rigorous quality standards by accurately measuring friability and abrasion,...

Multi-parameter tablet hardness tester for laboratories
Optimize your tablet production line with a versatile tester that...

Advanced dissolution tester for pharmaceutical laboratories
Achieve consistent and reproducible dissolution results with...

Manual dissolution tester for pharmaceutical applications
Achieve precise and repeatable sampling in pharmaceutical test...

Automated dissolution testing with direct Hplc injection
Streamline your lab operations with precise and efficient direct...

Integrated Uv-vis spectrophotometer for dissolution testing
Streamline your dissolution testing with real-time UV-Vis an...

Real-time Uv-vis analysis for dissolution testing
Efficiently perform dual dissolution tests simultaneously, seamlessly i...

Automated dissolution testing system for 16 vessels
Maximize your laboratory throughput by conducting parallel dissolutio...
Dissolution testing software for automated analysis
Streamline your laboratory testing with seamless data capture and in-...

Dissolution testing software for pharmaceutical industry
Optimize dissolution testing with advanced software that manages...
Automated sample preparation software
Enhance your laboratory’s efficiency with a software solution that automates sa...

Self-cleaning dissolution tester for automated series testing
Achieve unparalleled precision in dissolution testing with...

Ventilated autoclave for sterilization processes
Optimize sterile production with advanced ventilated autoclaves, ensuring...

Benchtop Sip fermenter for bioprocessing applications
Optimize your bioprocesses with precise control over fermentation a...
Benchtop tangential flow filtration system
Ensure high-purity yields and streamline your filtration processes with this be...

Customizable pilot-industrial fermenters bioreactors
Designed for precision fermentation, these fermenters and bioreactor...

Medical lab sterilization autoclave
Ensure product sterility with high-performance ventilated autoclaves designed to optimi...

High shear lab mixer for laboratory work and r&d
Achieve unparalleled precision and consistency in laboratory and pilot-sc...

High shear batch mixer for industrial mixing
Optimize your production cycle with this high shear batch mixer, designed to ...

Laboratory freeze dryer for research and process amplification
Optimize freeze-drying processes with precision control, ...
Industrial grinding solution for hard and abrasive materials
Efficiently grind and mill a spectrum of materials from coa...

Loss-in-weight feeder for battery cell production
In battery cell production, achieving precise dosing and safe handling ...

Frozen storage and shipping for biopharmaceuticals
Ensure the integrity of critical biopharmaceuticals with a robust solu...

High-accuracy moisture analyzer for lab weighing
Achieve precise and reproducible moisture content determination for diver...

Explosion proof weighing cells for hazardous environments
Designed for hazardous environments, these weighing cells ensu...

Lithium-ion battery powder processing solution
Optimize your lithium-ion battery manufacturing with advanced powder treatm...

Pharmaceutical horizontal batch centrifuge
Ensure unparalleled product purity and operational efficiency with a centrifuge...

Continuous hot air conveying dryer for chemical and pharmaceutical industries
Ensure rapid moisture removal with high-...

Semi-continuous freeze dryer with easy-loading
Freeze-drying is both a time- and energy-consuming process. In addition to ...

3D powder mixer for active pharmaceutical ingredients
Proper mixing and homogenization are essential to achieve a reliabl...

Agitator bead mill reactor for molecular synthesis by mechanochemistry in continuous flow
To achieve cleaner, safer,...

Agitator bead mill for API
To achieve precisely defined API properties and safe and reproducible production, rigorous implem...

Continuous wet granulator and dryer for R&D
Pharmaceutical laboratories need compact equipment to handle and produce small...

Dry granulator for pharmaceutical powders
In pharmaceutical industries, where large-volume production is the norm, compact...

GMP dispersing machine for liquid formulations
Multi-staged rotor/stator mixers generate the high shear forces required to...

Industrial sieving machine for pharmaceuticals
One of the physical attributes that granular pharmaceutical products must h...

Fluidized bed system for the granulation and drying of pharmaceuticals
Pharmaceutical industries employ several process...

Continuous roller compactor for dry granulation of pharmaceuticals
When making tablets, pharmaceutical manufacturers ne...

Container blender for pharmaceuticals
Manufacturing pharmaceutical solids like tablets requires using a proper blender to b...

Pharmaceutical tablet coating machine
Tablet coating requires spraying, mixing, and drying procedures that must be carried ...

Sterile Filtration System for GMP-grade products
Processing equipment used in industries regulated by GMP must ensure full...

Single-use containment valves
From GMP and HSE perspective material transfer is critical for chemical and pharmaceutical for...

Big bag filling machine
Active pharmaceutical ingredients demand high handling standards to prevent cross-contamination. More...

High containment micronizing isolator
Powder processing for chemical compounds demands product-specific micronizing solutio...
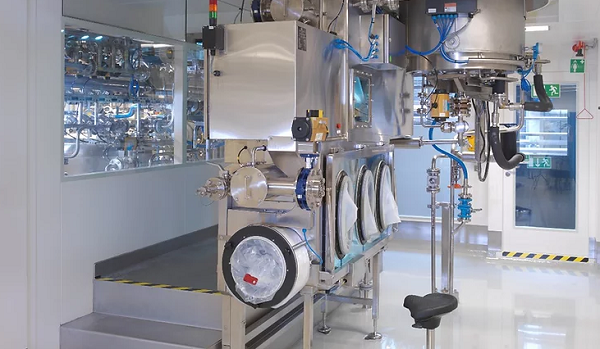
Filter dryer discharging isolator
Contained discharging is critical to the quality of filtration and drying processes. But ...
Accurate multiple dosing system for powders
Dosing multiple powders in a batch process is time inefficient, especially whe...
Powder micro-dosing system
Pharmaceutical powders in metered doses demand maximum filling precision. This is particularly ch...

API containment isolator
Contamination is a major concern in powder handling, not least when processing pharmaceutical produc...

Powder transfer system
Loading powder from a container source to vessels, tanks, or mixers that are under pressure may be haz...

GMP homogenizing system
Manufacturers need production mixing equipment that is capable, flexible, and easy to maintain. Accur...

Inspection system for tablets chemical composition
The packaging of pharmaceuticals and oral solid products such as table...

Fluid bed dryer for pharmaceutical solid dosage form
Fluid bed drying is a pharmaceutical process carried out to reduce t...

Industrial high shear mixer granulator for drug formation
It is rather difficult to successfully produce a pharmaceutica...
Hygienic floor scale
The food and pharmaceutical industries require maintaining high levels of sanitations as well as logisti...

Hygienic mobile scale
Having a reliable and accurate weighing and measuring solution is absolutely essential, especially in t...

Fast gravimetric powder microdosing 50 - 200 g
Micro-dosing powders of high-value food or chemical products can be challen...

Gravimetric powder microdosing 2 - 100 g
Powders and granules are commonly dosed for the manufacturing of pharmaceuticals, ...

Gravimetric powder microdosing 0.400 - 2 g
If you need to process high value food, pharmaceuticals or chemicals, there is ...

Single-use powder handling bag
Containers for handling pharmaceutical and biopharmaceutical powders may be disposable, to av...

API powders storage bottle
Users who prefer rigid storage over single-use pharma charge bags, require lightweight solutions ...

Single-use containment valve
Safe transfer of liquids and hazardous powders into systems can be a challenge in pharma, bioph...

High containment closing system
Pharma and biopharma laboratories need fast effective solutions for tightly sealing bags for...

High containment sampler
Drawing a sample for evaluation from a running process is not a simple task, especially when dealing...

Metal detector for pharmaceutical powders
Powdered and granulated pharmaceuticals require careful screening against metal ...

Laboratory scale active freeze dryer
The laboratory-scale active freeze-drying is used for dehydrating high-value products ...

Entry level HFFS Machine for flat sachets
Traditional horizontal form fill and seal sachet machines for lower volume lines...

Flexible containment docking solution
When sampling or transferring hazardous substances and sensitive bulk goods, a dust-t...

Flexible containment zip
Transfer of sensitive and toxic bulk goods across process units requires specialist handling to prev...

Drum conditioner
Many pharmaceutical production processes involve the use of products that have naturally hardened within thei...

Pharmaceutical container lifter
Moving larger or heavier containers from height is out of the scope for standard handling eq...

Vacuum sack lifter
In the production of pharmaceuticals and other high-hygiene environments, manual handling of inputs or int...

Container lifter
Container handling in the pharmaceutical industry brings its own set of unique challenges. Traditional barrel...

Mobile drum lifter
The production of pharmaceuticals and other hygienic products often requires the handling of large and bul...

Powder transfer vessel
Intermediate bulk storage of pharmaceutical product requires specialist containers. For increased effi...

GMP Jib crane
Vacuum manual handling systems are the ideal way of quickly and safely moving small to medium sized loads in all...

Cleaning and sterilization systems
An increasingly vital part of high quality pharmaceutical production is a dedicated Clea...

Contained capsule filler
For pharmaceutical formulations that are highly active specialized capsule filling equipment is ofte...

Tumbler sieve for classifying and dedusting granular materials
For the classification of delicate granular material, scr...

Small feeder with flexible wall hopper
In many laboratory applications and production processes, smaller quantities of powd...

Checkweigher for sachets and sticks
The checkweighers for multiple lanes have a 1.5 times faster response speed and 2 times...

Conical mill for drug formulation
The efficient size reduction of granular powders is a key step in many pharmaceutical pro...
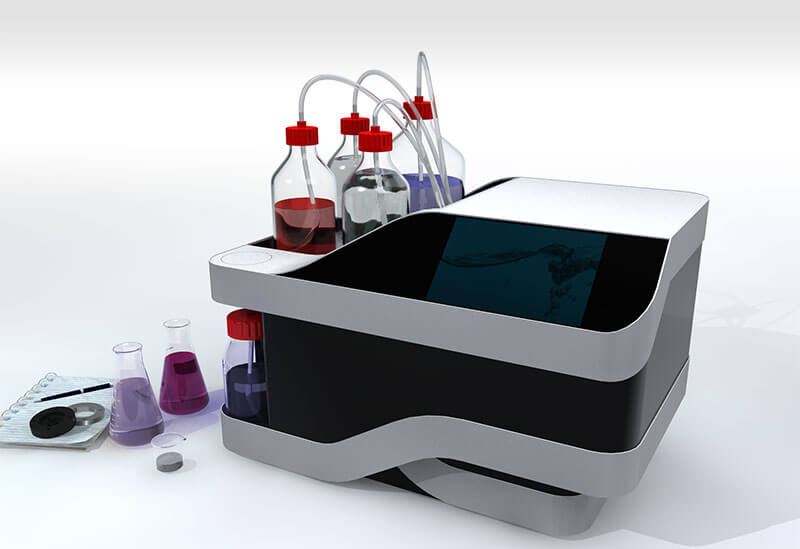
Microencapsulation system for your drug delivery system
Whether you want to improve the stability of nutrients, protect a...

High capacity capsule filler
In the highest volume pharmaceutical production environments, capsule filling needs to be extre...

High-shear mixer
For the efficient mixing of larger batches of pharmaceutical compounds and high-shear mixer provides the opti...

GMP laboratory freeze dryer
Freeze dryer designed for aseptic and small-scale production of high-value products.

Magnetic agitator
Reliable mixing and agitation are essential to any quality production process that involves a fluid. This n...

Centrifugal disc filter
The pharmaceutical and bioprocess industries need filtration processes of particularly high quality t...

Spherical dryer
Drying vessels that are simple, reliable, and easy to maintain increases product quality and process efficienc...

Filter dryer
The pharmaceutical and bioprocess industries need filtration processes of particularly high quality to ensure the...

Transfer system for high-containment environments
A high-performance transfer port system that combines ultimate safety, ...

Purified water systems
Highly Purified water is used in the preparation of medicinal products where bacterial endotoxins need...

Half-suit tester
Designed to allow operators to perform sterility testing in an aseptic environment providing assurance of mat...
Glove tester
Testing device of the containment capability of gloves, which is as important as the other parts of the integrate...
Rotary vacuum dryer
Powdered products tend to agglomerate during vacuum drying. This adds an additional step to the productio...

Premium vacuum conveyor
When you have a need to tailor make your conveyor and still have the high requirement on hygiene, e.g...

Multipurpose and R&D freeze dryer
A multipurpose unit in the research and development field. Some of the most common applic...

Hygienic automatic iris valve
In automated systems where controlled discharge of powders and bulk solids is required with a ...

Hygienic hand-operated iris valve
Hand-operated valves are required for the walls of clean rooms for glanding around cables...
Slide gate valve
In the powder and bulk handling industries, in-line shut off valves are required to provide uninterrupted smo...

Roller gate valve
In large scale operations, modular and configurable valves are required to isolate and control dry powders,...

Horizontal vacuum dryer with eccentric agitator
Conventional dryers are inefficient and can lead to significant mechanical...

Horizontal paddle vacuum dryer
Agitated vacuum dryers can be difficult to clean which makes them unsuitable for multi-produc...

Laboratory-scale vacuum tray drying oven
Vacuum drying is used to remove moisture from sensitive materials. Drying small ba...

Bellows for pharmaceutical powders
Thermal and mechanical changes can lead to stresses in industrial piping systems. These ...

High containment split butterfly valve
Powder containment is the cornerstone of chemical and pharmaceutical manufacturing. ...
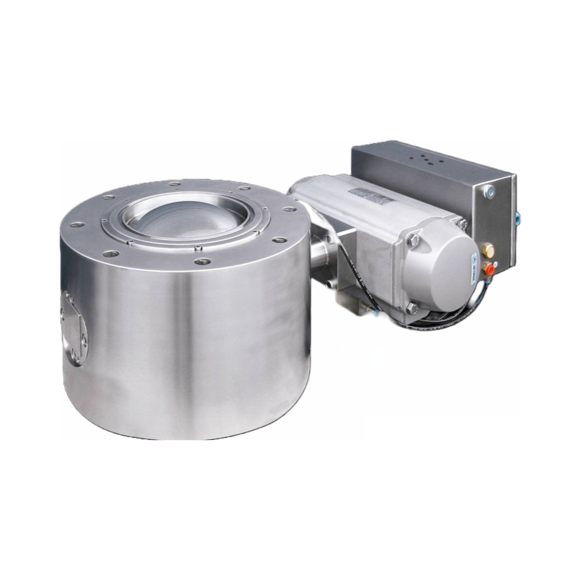
High containment segment ball valve
Valves fitted in blenders and dryers can have dead spaces that prevent complete process...

Removable GMP compliant connections for tubing
Tubing frameworks can have rough welding joints, corners and sharp angles t...

Negative pressure isolator
Used for products biologically hazardous, that also require minimized cross contamination.

Downflow booth
Provides the highest levels of operator protection from potentially harmful airborne contaminants generated dur...

Vertical laminar flow cabinet
HEPA filtered vertical laminar airflow (down flow) creates an ISO 14644-1 (Class 5) work area ...

Horizontal laminar flow benches
HEPA filtered horizontal laminar airflow (crossflow) creates an ISO 14644-1 (Class 5) work a...

Positive pressure isolator
Ideal for products which have no biological risk but require high sterile conditions.
Hygienic floor scale with lifting device
The food and pharmaceutical industries require maintaining high levels of sanitati...

Mobile pallet turner
Turning your pallet may allow for more effective pallet changing than the conventional lifting and grabb...

Flexible contained powder charging solution
Filling of transportation bags and discharging them into mix tanks or reactor ...

Flexible contained charging for split butterfly valves
Poly bottles with split butterfly valves are often used to transfe...

In-line pallet changer
When you need to transfer load from one pallet to another without tilting or turning, then selecting t...

Non-tilting pallet changer
Stable vertical lifting of the load from the pallet to enable EPAL or industrial pallet exchange.

V-shaped pallet changer
If you are looking for a sturdy pallet changing solution, a stationary V-shaped pallet changer can be...

Pallet changer floor level
For transferring goods from wooden pallets to plastic pallets, or vice-versa, this Pallet changer...

Pallet changer
For turning loads and exchanging them on pallets, here is a stationary pallet changer that will load and unload...

Mobile pallet changer without turning
If you are looking to change the pallet without turning the load, through a lateral p...
Versatile mobile pallet turner
Here is the versatile mobile pallet turner that completes the typical features of all mobile ...

Mobile pallet turner for higher stacks
When you need to opt a pallet changer that is capable of lifting and turning loads a...

Aseptic barrier systems
Designed for sterile product and Potent API’s either with low or high OEL requirements.

Restricted-access barrier systems
Create a physical barrier between the operator and the product. More flexible than an iso...

Flexible contained handling of powder transfer bags
Filling of transportation bags and discharging them into mix tanks or...

Crimping system
Flexible disposable containment systems in the form of specially designed plastic bags are an excellent choice...

Flexible contained drum transfer system
Transferring powders from drums to process equipment such as reactors or mills can ...

Flexible contained powder discharge into FIBC’s
Flexible intermediate bulk containers known as FIBC’s are commonly used in...

Flexible contained powder discharge into drums
Powdered products are handled every day in the pharmaceutical and bioproces...

Horizontal chemical scraper centrifuge
For slurries difficult to discharge, a scraper can improve process reliability, effi...

Horizontal pharmaceutical scraper centrifuge
For slurries difficult to discharge, a scraper can improve process reliabilit...

Isolator centrifuge
For transferring highly active pharmaceutical ingredients (HAPI) without contamination from the isolator ...

Mobile pilot plant centrifuge
The system is perfectly suited for pilot plants as well as small-quantity productions in the f...

Vertical top discharge centrifuge
Discharge solids vertically upward through manual action, optionally using a filter bag o...

Vertical peeler centrifuge
A vertical peeler centrifuge is used to separate solids, usually to separate fine particles from ...

Vertical scraper centrifuges
Vertical Bottom discharge Centrifuges work discontinuously, discharging the solids to the botto...

Pusher centrifuge
Pusher centrifuges are continuously operating filter centrifuges and can have several basket stages dependi...

Checkweigher for stand-up pouches
When working with several lines of stand-up pouches, you can benefit from controlling the...