Automatic powder vacuum conveying system for infusion production
Enhance your production line with our vacuum conveying solution, designed to streamline powder transport and minimize contamination risks while ensuring precise, automated weighing and feeding for complex batch production.
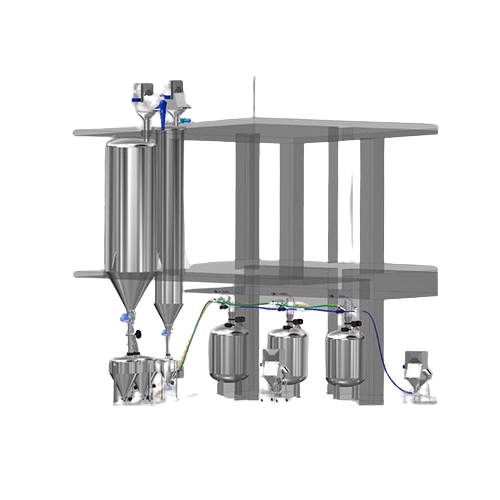
Conveys and Weighs Powders for Sterile API Production
The Powder Vacuum Conveying System for Large Volume Infusion Lines from AUSTAR is a sophisticated solution designed to streamline powder handling in pharmaceutical and chemical production environments. This system employs a closed, dust-free vacuum transport mechanism, significantly reducing labor intensity and eliminating dust leakage in clean rooms. Ideal for conveying and precisely weighing pharmaceutical granules, lyophilized powder formulations, sterile penicillin, and fine chemical intermediates, it integrates seamlessly into existing lines for sterile API and biopharmaceutical production.
The system boasts robust automation through a BATCH control system conforming to S88 standard, facilitating flexible production across various product batches while ensuring compliance with FDA, cGMP, and 21CFRPart11 standards. AUSTAR’s design includes automatic pipeline switching to enhance production efficiency and minimize cross-contamination. Moreover, its compact design places critical components strategically to lower air conditioning demands and energy usage in production areas.
Equipped with both CIP and SIP features, the system allows for rapid cleaning and sterilization, dramatically reducing turnaround times between batches. Its integration capability supports existing processes with ease, guaranteeing a smooth fit within the operational structure of pharmaceutical and biotech plants. Additionally, the system is available in high-grade materials suitable for demanding pharmaceutical standards, ensuring durability and long-term reliability.
Benefits
- Enhances production efficiency by automating powder conveying and weighing, reducing manual labor.
- Minimizes cross-contamination risks with its closed transport and automatic pipeline switching system.
- Lowers energy consumption through compact design, optimizing clean room air conditioning requirements.
- Ensures compliance with pharmaceutical standards with fully automated BATCH control system.
- Shortens batch conversion times with integrated CIP and SIP capabilities.
- Applications
- Sterile api production, Fine chemicals, Pharmaceutical powder, Biopharmaceuticals, Non-sterile api production
- End products
- Active pharmaceutical ingredients (apis), Monoclonal antibodies, Fine chemical intermediates, Pharmaceutical granules, Sterile penicillin, Lyophilized powder formulations
- Steps before
- Raw Material Preparation, Weighing, Centralized Preparation
- Steps after
- Batch Conversion, Production, CIP/SIP Process
- Input ingredients
- raw materials, powder, large volume infusion
- Output ingredients
- accurately weighed powder, batch production, multiple product batches
- Market info
- Austar is known for specializing in the design and manufacture of engineered-to-order industrial equipment, particularly in the pharmaceutical and biotechnology sectors, offering solutions that focus on quality, innovation, and meeting specific customer requirements.
- Transport Mode
- Closed and dust-free
- Operation Control
- Automatic
- Weighing Accuracy
- Automatic, accurate
- Cross Contamination Risk
- Minimized
- CIP/SIP
- Available
- Batch Conversion Time
- Greatly shortened
- Space Design
- Compact
- Tank Placement
- Unclassified area
- SKID Placement
- C-class area
- Air Conditioning Consumption
- Reduced
- Information Management System
- BATCH control system
- Compliance
- FDA, cGMP, 21CFRPart11
- Production Line
- Single, flexible
- Process Confidentiality
- Ensured
- Production Efficiency
- Improved
- Working mechanism
- Vacuum conveying system
- Integrated steps
- Preparation, feeding, weighing, transporting
- CIP/SIP
- CIP and SIP capable
- Batch vs. continuous operation
- Batch control system
- Automation level
- Automatic
- Changeover time
- Reduced batch conversion time
- Cross-contamination prevention
- Closed and dust-free transport mode
- Density/particle size
- 0.5–2.5 g/cm³
- FDA Compliance
- 21 CFR Part 11
- GMP Compliance
- cGMP
- S88 Compliance
- S88 Standard
- Machine footprint
- Compact
- Tank placement
- Unclassified area
- SKID placement
- C-class area
- Design
- Lean SKID
- Transport mode
- Closed and dust-free
- Pipelines
- Automatic switching
- CIP/SIP capability
- Yes
- Control panel type
- Batch control system, S88 standard
- Integration possibilities
- FDA, cGMP, 21CFRPart11 compliance
- Automation level
- Automatic, PLC-controlled




