Sublimation front monitoring system for freeze drying
Ensure precise control over your freeze-drying process with real-time sublimation front monitoring, optimizing drying phases while maintaining critical product quality attributes and reducing cross-contamination risks.
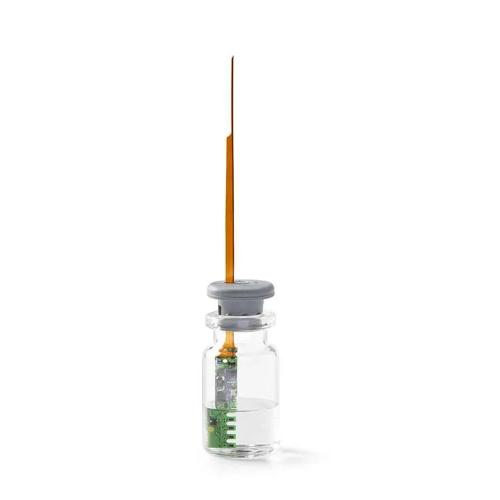
Monitors Sublimation Front Advancement in Real-Time
The Telstar Sublime is a real-time sublimation front monitoring system designed for pharmaceutical and biotechnology industries. This innovative system employs single-use wireless probes to track the sublimation front temperature interface during freeze-drying. It excels in optimizing cycle development by providing accurate multipoint temperature readings, thus enhancing process understanding and repeatability. Key applications include the production of monoclonal antibodies, lyophilized vaccines, and vitamin tablets. The Sublime integrates seamlessly with automatic loading and unloading systems, reducing logistics complexity and cross-contamination risks. It is fully compliant with cGMP and FDA 21 CFR Part 11 standards, ensuring the highest level of quality control for your operations.
Benefits
- Enhances process robustness and repeatability with precise real-time monitoring.
- Simplifies logistics and reduces contamination risks with single-use wireless probes.
- Supports regulatory compliance with cGMP and FDA 21 CFR Part 11 standards.
- Improves operational efficiency by integrating with automatic systems.
- Facilitates accurate cycle development and validation for quality assurance.
- Applications
- Vitamins, Pharmaceutical tablets, Pharmaceutical powder, Biopharmaceuticals, Biological products
- End products
- Lyophilized vaccines, Monoclonal antibodies, Penicillin tablets, Probiotic supplements, Vitamin c powder
- Steps before
- Formulation, Dispensing, Vial Loading
- Steps after
- Unloading, Packing, Quality Control, Storage
- Input ingredients
- freeze-drying process, vial format, product for primary drying phase
- Output ingredients
- sublimation front data, temperature readings, freeze-dried product, process product temperature data
- Market info
- Telstar is known for specializing in the design, engineering, and manufacturing of advanced vacuum and aerospace solutions, gaining a strong reputation for their innovative and customized equipment in pharmaceutical, medical, and scientific research sectors.
- Temperature Sensors
- 5 independent sensors
- Reading Type
- Accurate multipoint temperature reading
- Communication
- Wireless
- Probe Type
- Single use disposable probes
- Power
- Battery-free
- System Compatibility
- Compatible with fully automatic loading and unloading systems
- Vial Compatibility
- Compatible with vial format freeze-drying
- Control System
- Integrated in Freeze-dryers SCADA
- Sterilization
- Pre-sterilized ready-to-use probes
- Real-time monitoring
- Enabled
- Sublimation front monitoring
- Wireless probes
- Temperature sensors
- 5 independent sensors
- Automation level
- SCADA integrated
- Loading and unloading compatibility
- Automatic systems
- Pre-sterilization
- Ready-to-use probes
- Density/particle size
- 0.5–2.5 g/cm³ / 50–1000 µm
- Vial Format Compatibility
- Yes
- GMP Compliance
- cGMP compliant
- FDA Compliance
- FDA 21 CFR part 11 compliant
- Control panel type
- Integrated in Freeze-dryers SCADA
- Discharge method
- Automatic loading and unloading
- Probe type
- Single use disposable
- Sensor quantity
- 5 independent temperature sensors
- Control panel type
- Integrated in SCADA
- Integration possibilities
- Compatible with vial format freeze-drying








