
Let's make healthcare products
Find innovative healthcare products equipment and connect directly with world-leading technology suppliers
Healthcare products are goods or services used to improve, maintain or prevent the deterioration of health or ease the consequences of illness. Healthcare products include a wide range of goods, from hospital clothing, over plasters, wound care products and diagnostic devices. These products must be clean and contamination-free, which is achieved with strictly regulated healthcare equipment.
Top picks for healthcare products

Filling solution for consumer healthcare products
Navigate complex liquid product formulations with a versatile filling s...

Case packer for pharmaceutical and healthcare products
Streamline your packaging line with a versatile case packer design...

Flexible web converting system for healthcare products
The market for web-based goods moves fast and may outpace the manu...

Modular assembly line for healthcare products
Assembly setups need to be constantly rethought and reconfigured to adapt qu...
What are you making?
Cardiac stents

Bandages

Baby wipes

Blood glucose test strips
Yttrium-90 microspheres
Zirconia dental crowns

Wipes
Whitening toothpaste
Test kits
Surgical scissors
Surgical masks

Surgical gloves
Stents
Protein assays
Medical testing kits
Ostomy
Orthopedic implants

Oral care

Mouthwash
Implants

Implantable medical devices

Hygiene products

Household appliances
Hip implants

Hand sanitizer
Endoscopes

Fluoride toothpaste
Disposable syringes

Disinfectants

Diagnostics
Diagnostic reagents
Diagnostic kits

Eye care products
Dental
Animal health products
Animal diagnostics
Covid-19 test kits
Coronary stents

Continence care

Coated lenses

Cleaning wipes

Chinese traditional medicine
Ophthalmics
Sublingual spray

Wet wipes

Medical gummies
Contact Lenses

Medical devices

Wound care products

Diagnostic devices

Inhaler blisters

Plasters
Tell us about your production challenge
Which healthcare product equipment do you need?
As healthcare products entail a wide range of goods, the production processes vary considerably depending on the product type. However, they all undergo strict regulations regarding sterility; therefore healthcare equipment such as steam and air mixture sterilization autoclaves as well as GMP autoclaves, is crucial in healthcare products production.
Furthermore, the products must be safely packaged. Appropriate machinery includes automatic medical forming and sealing blister machines for packaging goods such as capsules and tablets. Although production processes vary depending on what you are making, one of the most common processes used in medical device production is injection molding, which is suitable for various materials.
Plasters and wound-care products: production of wound dressings
Skin surface wounds of various severities must be treated with wound care products such as plasters and other wound dressings. Most band-aids consist of an absorbent pad covered by woven fabric, plastic, or latex rubber as an adhesive. The production starts with a thin coat of glue being applied on one surface of the fabric and heated to roughly 49°C. The fabric is cut into varying sizes, put into a large roll, and sealed with a small cushion pad.

Other types of wound care products include modern wound dressings that come in the shapes of passive dressings made of synthetic polymers or active dressings that come in the shapes of foams, hydrogels, etc. Another modern wound dressing is bioactive dressings made from biomaterials which influence the healing process due to collagen as it initiates fibroblast formation and accelerates endothelial migration upon contact with wound tissue.
Liquid healthcare products: eyedrops, eardrops, ampules and vials
Eyedrops and eardrops are liquid medical products that are applied directly onto organs to treat inflammations, infections, and diseases. The formulation starts with preparing a solution that contains preservatives, antioxidants, stabilizers, and other required ingredients, which is then clarified and sterilized. Due to the direct contact with organs, the crucial step in manufacturing ear and eyedrops is using purified and sterile water, as well as modifying viscosity and pH. Ampules and vials, on the other hand, are plastic or glass containers for liquid medicine. Therefore, specialized ampule making equipment should be made from high-quality stainless steel because the contents must avoid contamination.
The production of both products necessitates specialized liquid filler machines, such as aseptic vial filling equipment, to ensure safety and quality as it removes harmful pyrogens, strict sterilizing processes, and labeling machines. Sterilization is essential as any microbial contamination could result in disease transmission. Finally, using fully or semi-automatic inspection machines with image sensors and magnifying cameras guarantees the outright safety of the finished product.
The delicate process of packing and cartoning healthcare products
When packing vials, ampoules or medical devices into boxes, it is necessary to handle them carefully to prevent breaking. Furthermore, cartoning machines must be sterile and hygienic to prevent cross-contamination. The cartoning control is ensured by a robot-supported automated process to ensure complete control, reliability, and low rejection rates. The healthcare product is unloaded onto the device to the conveyor belt, and the microcomputer will transmit the command to the suction cup to unload the box. Once the box is opened and stabilized, the push rod places the product into the box. Both the product and carton must be handled carefully to prevent damage to delicate materials. Finally, the box is closed by the mechanism using hot melt.

Quality and regulations of healthcare products
Although standards for healthcare products can vary from country to country, WHO works to develop globally standard norms and guidelines for quality, safety, and efficacy. At the regional level, WHO supports countries in building regulation systems that promote certain standards. For instance, FDA’s regulations for healthcare products entail informative labeling, reports of device-related injuries, having the manufacturing business registered with the FDA, and ensuring that the device is effective and finished. In the EU, standard rules are provided for conducting clinical trials to test the safety and efficacy of healthcare products under controlled conditions. The authorization is based on three key criteria: quality, safety and efficacy.
Which healthcare products technology do you need?

Case packer for pharmaceutical and healthcare products
Streamline your packaging line with a versatile case packer design...
Filling system for consumer healthcare products
For small to medium productions needing versatile filling and sealing, thi...

Filling solution for consumer healthcare products
Navigate complex liquid product formulations with a versatile filling s...

Flexible web converting system for healthcare products
The market for web-based goods moves fast and may outpace the manu...

Modular assembly line for healthcare products
Assembly setups need to be constantly rethought and reconfigured to adapt qu...

Digital inline printing system for pharmaceutical blister packaging
Enhance production flexibility with a high-speed, d...

Liquid dispensing isolator for sterile environments
Maintain product sterility by utilizing a liquid dispensing isolator ...
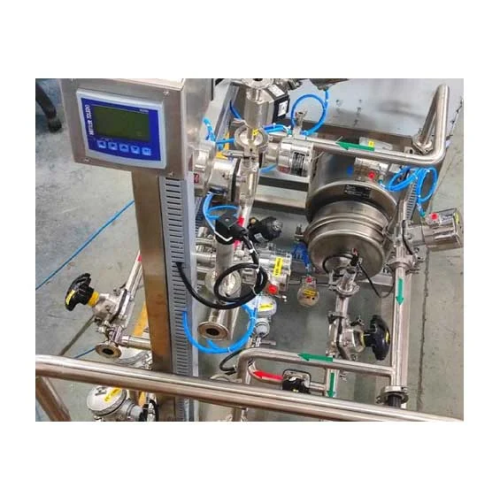
Cip & Sip systems for pharmaceutical cleaning
Ensure effective cleaning and sterilization in production lines with customi...

Intermittent stick pack packaging system for pharma products
Achieve precise dosage and packaging of pharmaceutical and ...

Intermittent stick pack system for pharmaceuticals
Effortlessly streamline your packaging process with a high-speed, mult...

Flat pouch towelette packaging system
Optimize your packaging line for high-speed towelette production with this system, de...

Hygienic Cartoning Machine For Pharma and Health Products
When packing single or multiple vials, ampoules or devices int...

Liquid jet mixer for homogeneous tank mixing
Achieve efficient and consistent mixing in large storage tanks with this adva...

Clarifier for beverages
Enhance beverage clarity and purity with high-performance centrifugal separation, expertly designed t...
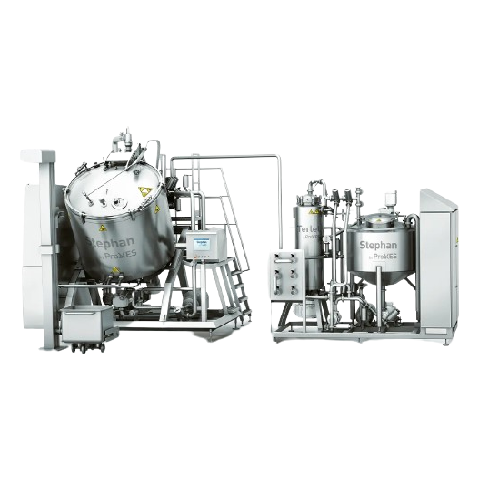
Continuous caramel production line
Optimize your caramel production with seamless integration, achieving precise fat meltin...

Continuous ultra-high temperature processor for processed cheese
Ensure precise thermal treatment and enhanced flavor re...

Continuous heat exchanger for soups and sauces
Optimize your soup and sauce production with a continuous heat exchanger th...

Tube filling solution for plastic and laminate tubes
Ensure precise tube filling and sealing with quick changeovers, enha...

Tube filling solution for plastic, laminate, and aluminum tubes
Optimize your production line with a versatile tube fill...

Tube filling station for plastic, laminate, and aluminum tubes
Efficiently seal and fill various tube materials, ensurin...

Compact automated nucleic acid extraction system
Quickly automate nucleic acid purification to reduce hands-on time and mi...

High-throughput tube transfer system for lab automation
Streamline laboratory workflows by automating high-throughput tub...

Full cube ice makers for commercial use
Effortlessly produce perfectly shaped ice cubes with high efficiency, ideal for mai...

Horizontal cartoner for diverse industries
Streamline your packaging process with precise cartoning capabilities, effectiv...

Outsert application system for pharma bottles
Effortlessly enhance packaging efficiency in pharmaceutical lines with this ...

Outsert applicator for bottles and cartons
Enhance the efficiency of your packaging line by seamlessly applying informatio...

Vacuum filtration for sterile media and buffer preparation
Achieve reliable sterilization and filtration of cell culture...

Sterilization in place fermentor for bioprocess applications
Optimize your fermentation processes with seamless integrat...

Automatic sachet filling and sealing system for liquids and creams
Optimize your cosmetic and personal care production ...

Automatic syringe and safety device assembly system
Optimize syringe and safety device assembly with precision and reliab...

High capacity filling line for jars and bottles
Need to streamline your packaging line? This high-capacity solution proces...

Industrial robot for low-payload applications
Optimize your production line with versatile automation solutions that enhan...

Flexible industrial robot for medium payloads
Optimize your production line with a versatile robotic solution that adapts ...

High payload industrial robot
Optimize your production with high payload robots designed to enhance flexibility and reduce o...

Compact palletizing robot for efficient material handling
Optimize your production line with a high-speed palletizing ro...

Autonomous mobile robot for flexible production
Enhance your production line with mobile precision; navigate and adapt to ...

Hydroalcoholic gel mixing system
Ensure seamless production of hydroalcoholic gels with an advanced mixing system designed f...

Linear vial washer for injectable drug containers
Achieve sterile conditions by efficiently removing contaminants from va...

Wet wipes converting line
Efficiently convert a variety of wet wipes with flexible settings and minimal machine downtime, en...
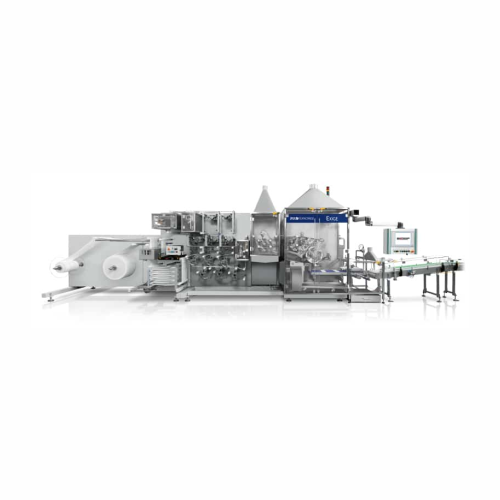
Wet wipe converting line for high-speed production
Optimize wet wipe production with a high-speed system that delivers pr...

Wet wipe converting line
Optimize your production with a versatile and precise solution designed for high-quality wet wipe ma...

Industrial pick and place system for packaging
Streamline your packaging line with precise robotic pick and place operatio...

Compact full product inspection system for pharmaceuticals
Easily detect fill level inaccuracies, closure misalignments,...

Liquid filling system for medical and pharmaceutical products
Enhance your production capabilities with high-speed liqui...

Flexible horizontal cartoning solution for various products
Streamline your packaging process with this versatile carton...

Sterile filling system for Iv bags
Ensure precise and sterile filling of IV and infusion bags with flexible, semiautomatic ...

Liquid dosing and assembly platform for pharmaceutical products
Optimize your production line with a seamless solution f...

Uv Dod Cmyk label and packaging film printer
Achieve high-precision, on-demand printing for narrow-web packaging films and...

Single-color inline digital printer for blister-lidding foils
Streamline your pharmaceutical packaging with precision pr...

Uv flexographic printing system for blister-lidding foils
Achieve precise and consistent single-color prints on blister-...

Iv bag flexo printer for pharma applications
Ensure precise and efficient printing on IV and chamber bags with a high-spee...

Uv flexo printing system for blister-lidding foils
Effortlessly integrate precise and vibrant two-color printing directly...

Digital packaging printer for blister-lidding foils
Ensure precise branding on blister packaging with high-speed digital ...

Modular Uv printing system for pharmaceutical packaging
Enhance your pharmaceutical packaging with high-speed, precision ...
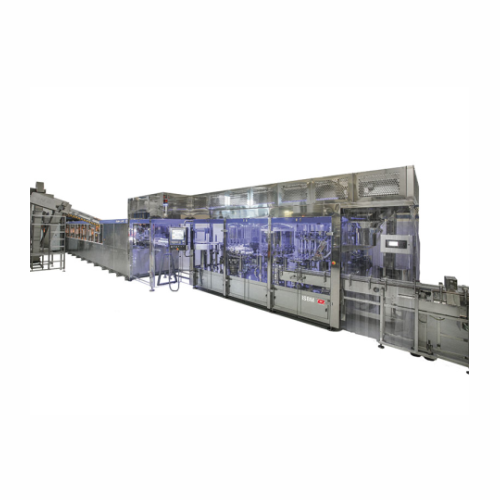
Injection stretch blow moulding line for pharmaceutical solutions
Optimize sterile solution production by forming, fill...

Optical inspection system for large parenteral containers
Ensure the quality and safety of liquid pharmaceutical product...

Sterile and nonsterile vial filling system for injectables
Achieve precision and safety in filling and stoppering with a...

Sterile and nonsterile filling for injectable solutions
Efficiently fill and stopper injectable solutions in both sterile...

Rotary washer for pharmaceutical containers
Ensure optimal preparation of pharmaceutical containers with advanced rotary w...

Vial capping solution for pharma applications
Efficiently secure the integrity of sensitive liquid medications with a flex...

Optical inspection for ampoules, vials, cartridges, and syringes
Ensure product integrity and safety with this advanced ...

Steam sterilizer for biomedical lab research
Ensure the highest level of biosafety and scientific integrity with this stea...

Turnkey bioreactor system for cell culture
Streamline your bioprocessing with a complete cultivation system; ideal for pro...

Stacking and bagging system for baby diapers
Optimize your production line with this high-speed solution designed for seam...

Adult incontinence products stacker and bagger
Optimize your adult incontinence product packaging with a high-speed stacke...

Stacker and bagger for baby diapers
Optimize your packaging line with high-speed diaper stacking and bagging built to strea...

Stacker and bagger for adult incontinence products
Optimize your adult incontinence product packaging with equipment desi...

Single track pallet conveyor for productions cells
Streamline your production operations with a versatile pallet conveyor...

Single track pallet conveyor for large items
Streamline your production with a versatile pallet conveyor system, designed ...

Twin track pallet conveyors for assembly and testing
Enhance your production line’s efficiency with a system that s...
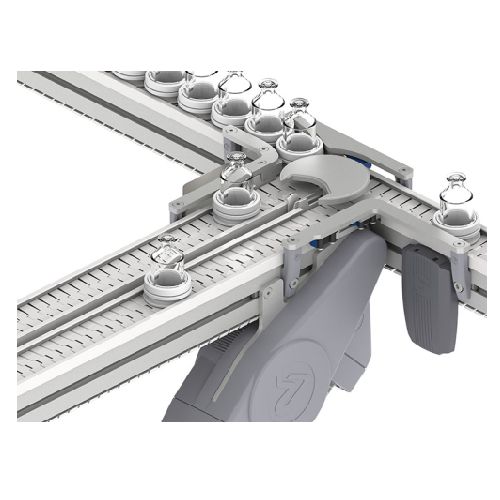
Conveying system for small, fragile products
Optimize your production line with high-capacity conveyors designed for effic...
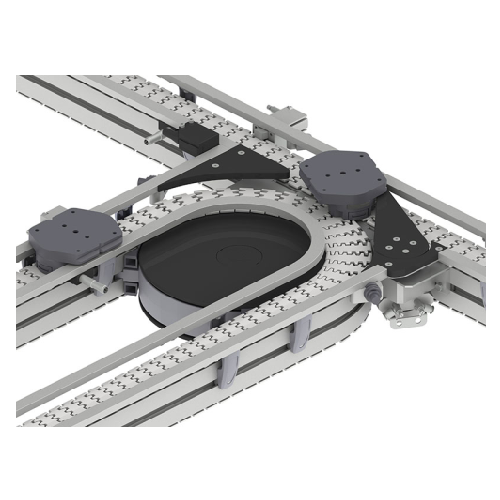
Single track pallet conveyors for electronics production
Efficiently manage single-piece flow with precise routing, balan...

Mobile isolator for aseptic environment transfer
Ensure aseptic conditions and seamless material transfer with this mobile...

Sterility testing isolator for aseptic environments
Ensure aseptic conditions and process integrity during sterility test...

Pharmaceutical turnkey project consultants
Streamline your biopharma production with turnkey solutions that cover everythi...

Vacuum transfer system for powders and granules
Effortlessly transfer and manage powders and granules with seamless integr...

Particle sizer for precise sifting
Optimize your production line with high-speed particle sizing, ensuring precise control ...

Cleanroom infrastructure for contaminant control
Ensure optimal contaminant control and regulatory compliance in cleanroom...

Modular cleanroom panel systems
Transform your cleanroom projects with modular panel systems that streamline installation an...

Chilled water plant for Hvac systems
Achieve precise temperature and humidity control in your production environment with a...

Pharma manufacturing process vessels
Ensure contamination-free production of sterile liquid formulations with process vesse...

Water for injections generation system
Ensure compliance with strict regulatory standards while providing pyrogen-free wate...

Single-use bioreactors for mammalian and insect cell cultivation
Optimize your bioprocesses with scalable, single-use bi...
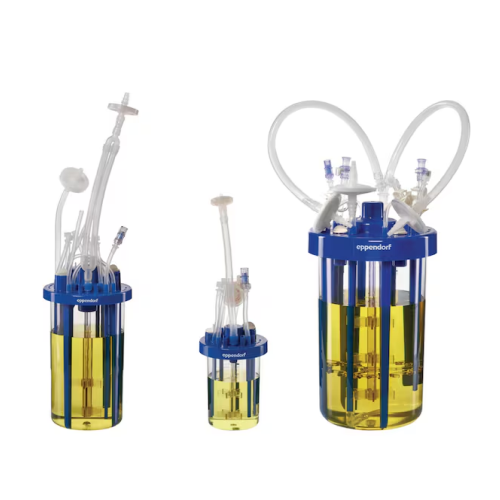
Single-use bioreactors for microbial fermentation
Optimize high-density fermentation with advanced single-use bioreactor ...

High-speed centrifuge for laboratory applications
Maximize efficiency in high-volume centrifugation tasks with this versa...

Horizontal flat pouching system for liquid soaps
Optimize your liquid packaging with high-speed, continuous operations tha...

Flat pouch packaging for towelettes
Design high-speed precision packaging to enhance towelette freshness and efficiency on ...

Flat pouch packaging for pharma products
For precise pharmaceutical packaging, achieve high-speed, intermittent flat pouchi...

Intermittent stick pack machine for pharma and healthcare
Looking to streamline your packaging operations with high-spee...

High-speed multi-lane flat pouch packaging system
Boost your packaging efficiency with a high-speed solution designed for...

Multi-lane vertical pouching solution for pharma industry
Achieve precision and high-speed efficiency in single-dose liq...

Intermittent side-load cartoner for pouches or stick packs
Optimize your packaging process with an intelligent side-load...

Horizontal flat pouching line for liquid soaps
Streamline your liquid product packaging with a dual-lane flat pouching lin...

Horizontal flat pouching line for towelettes
For manufacturers needing efficient packaging, our solution offers rapid hori...

Automatic mechanical line for wrapped cardboard boxes
Streamline your production of rigid set-up wrapped cardboard boxes ...

Automatic mechanical line for rigid set-up boxes
Elevate your packaging precision with a high-speed mechanical system tail...

Automatic mechanical line for small-size rigid cardboard boxes
Optimize your packaging line with a solution tailored for...

Automatic mechatronic line for rigid cardboard set-up boxes
Optimize your packaging line with this advanced mechatronic ...

Medium to large-size box making line
Streamline your packaging process with a versatile box making line that efficiently ha...

Digital box making line
Optimize your packaging capabilities with a versatile, digital box-making line designed to efficientl...

Water purification system for clinical diagnostics
Ensure consistent high-purity water supply for your critical clinical ...

Central water purification system for laboratories
Ensure consistent supply of ultrapure water essential for critical lab...

Centralized water purification system for laboratories
Ensure consistent purity and reliability for your laboratory proce...

Controlled rate freezer for biological and medical research
Ensure the integrity of biological samples while minimizing ...

Stainless steel work bench for radiochemistry labs
When handling radiopharmaceuticals, maintaining a contamination-free e...

Cell & gene therapy isolator with integrated incubation
Enhance your cell and gene therapy workflow with a modular isolat...

Hand-foot-clothing contamination monitor
Ensure precision in nuclear safety with advanced contamination detection for beta ...

Automatic compounding system for oncology drugs
Streamline sterile oncology drug preparation with this sophisticated compo...

Perspex syringe shield for beta radiation
Ensure the safety of your nuclear medicine operations with reliable beta radiati...

Horizontal cartoner for cosmetic applications
Perfect for packaging fragile cosmetic items, this cartoner efficiently mana...

Tube filling solution for health and beauty applications
Achieve consistent quality and efficiency in tube filling operat...

Shielded cells for radiopharmaceutical synthesis modules
Enhance your radiopharmaceutical production with precision-contr...

Small-scale gummy candy depositor
Optimize your confectionery production with this manual depositing machine, capable of pr...

Intermittent motion horizontal cartoner for large products
Simplify your packaging process with a solution that ensures ...

Bioreactors for cell culture processes
Efficiently cultivate sensitive cell lines with customizable bioreactors designed to...

Labeling solution for bottles and vials
Optimize your packaging line with this advanced labeling solution, designed to ensu...

Compact cleaning system for glass and plastic containers
Ensure exceptionally thorough cleaning and gentle handling of di...

Automatic bottle filling and capping line
Streamline your liquid product packaging with this high-speed solution, integrat...

Full-membrane water for injection (wfi) generation system
Ensure consistent high-quality injections by integrating real-...

Soft gel encapsulation system
Efficiently produce high-quality soft gel capsules with precision encapsulation, rapid drying,...

Integrated raw & auxiliary material pre-treatment system
Experience dust-free processing with an integrated system design...

High-efficiency high-capacity reactor for industrial processing
Enhance production efficiency with a reactor system desi...

General purpose freeze dryer for archaeological and biological samples
Preserve and restore cultural and scientific tre...

Controlled rate freezer for biological samples
Ensure the integrity of biological samples with precise temperature control...
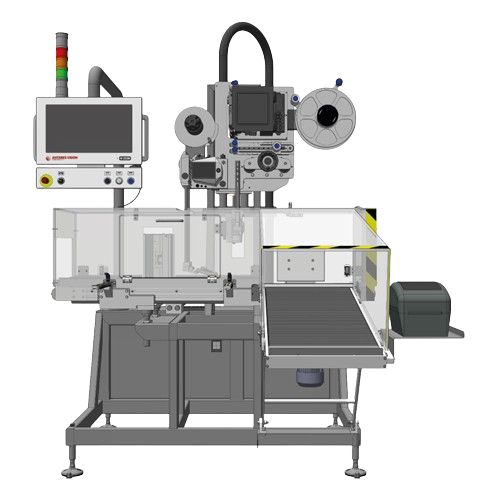
Standalone print & apply station for bottle and carton aggregation
Streamline your packaging operations by automating t...
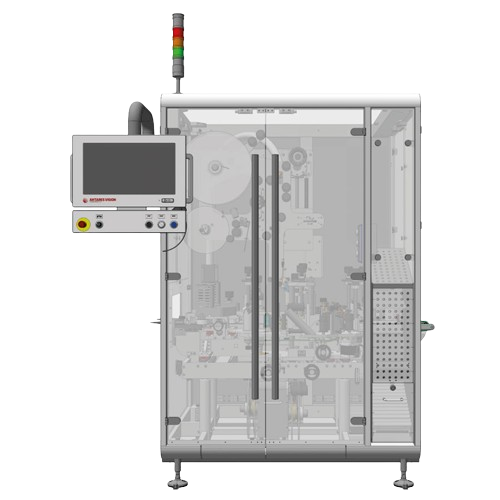
Standalone print & check system for cartons
Ensure data integrity and compliance in packaging operations with a versatile ...
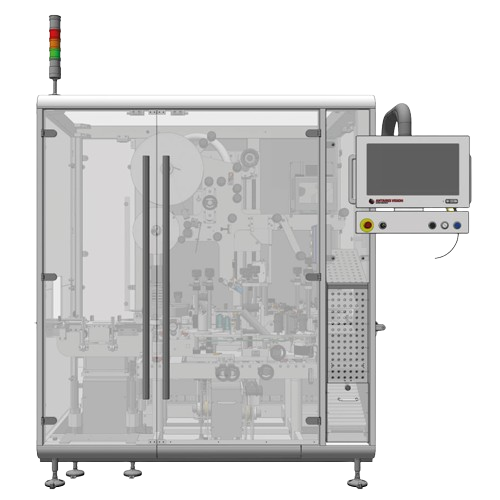
Print and verify cartons packaging systems
Streamline your packaging line with a versatile solution that prints, verifies,...

Checkweighers for medium-large product inspection
Ensure precise weight and quality compliance in high-speed production l...

Pillow bag horizontal form-fill-seal solution
Optimize production efficiency with seamless integration of continuous, high...

Spiral conveyor for logistics and packaging
Ensure seamless elevation and transportation of diverse products with this ver...

Modular conveyor system for internal unit load transport
Optimize your production line with a versatile conveyor system t...
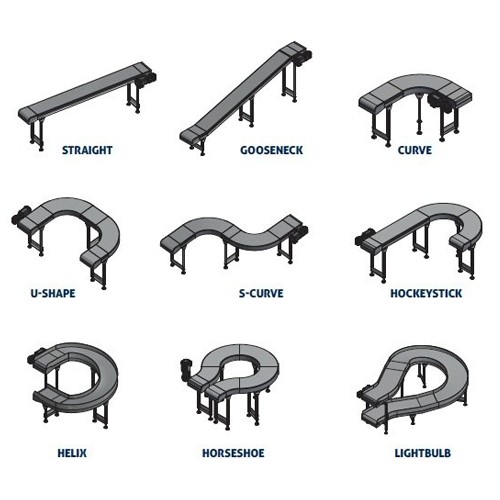
Curved conveyor for logistics and packaging
Efficiently tackle space constraints in your production line with a flexible c...

Controlled spiral conveyor for logistic centers
Efficiently manage parcel flow and minimize product damage with this spira...

Spiral conveyor for repeating size cases
Optimize your line’s efficiency by elevating rigid items at higher inclines,...

Rotary jet mixer for efficient liquid, gas, and powder mixing
Achieve seamless integration and efficiency in liquid, gas...

Tube feeder for pharmaceutical and personal care industries
Optimize your production line with this high-speed tube feed...

Autoclave for sterilizing medical instruments and containers
Achieve reliable sterilization for a wide range of medical ...

Ultra-low upright freezer -86°c for biological sample storage
Preserve vital biological samples with precise ultra-low t...

Ultra-low freezer for medical and biological storage
For critical preservation of sensitive samples, this ultra-low tempe...

Laboratory upright refrigerator for vaccine storage
Ensure the optimal preservation of critical vaccines and specimens wi...

Laboratory -40°c upright freezer
Ensure optimal preservation of sensitive biological samples with a freezer engineered for u...

Laboratory freezer for sample storage at -25°c
Ensure precise preservation of sensitive samples with an efficient upright ...

Liquid capsule filling for r&d and pilot-scale production
Optimize liquid capsule production with a compact machine that...

Ultrasonic tube sealer for plastic tubes
Seal plastic tubes with precision and speed using our ultrasonic technology, ensur...

Hygienic centrifugal pump for beverage applications
Ensure gentle handling and efficient transfer of sensitive liquids wi...

Cyclone tank for dust separation in production lines
Optimize your production line with efficient dust separation, ensuri...

Powder and granulate premixer
Achieve consistent, homogenous blends of powders and granulates with our advanced premixing te...

Industrial bucket elevator for efficient product conveyance
Enhance production efficiency by elevating and transferring ...

Direct discharge descender for product handling
Efficiently transport granulated materials without compromising their inte...

Lean phase vacuum conveying system for powder handling
Efficiently transport and separate powders and granules with preci...

Rotary tube selector valve for powder conveying
Streamline your pneumatic transport system with a robust solution that div...

Auger powder sampler for industrial quality control
Ensure precise quality control by integrating an auger powder sampler...

Pharmaceutical intermediate bulk containers (ibc)
Streamline pharmaceutical production with precise containment and trans...
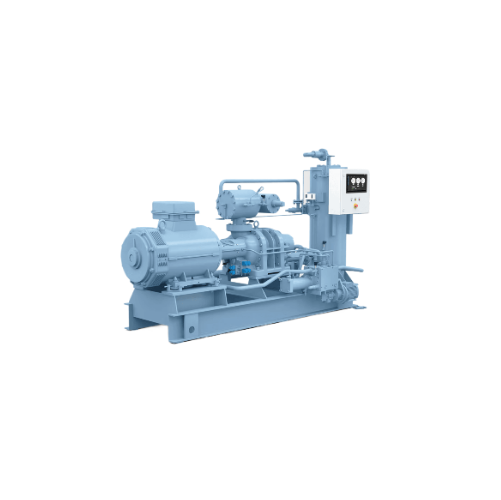
Single-stage screw compressor package
Optimize your refrigeration and heating processes with versatile packages designed to...

Screw compressor package for efficient industrial cooling
Optimize your cooling processes with a flexible, space-saving ...
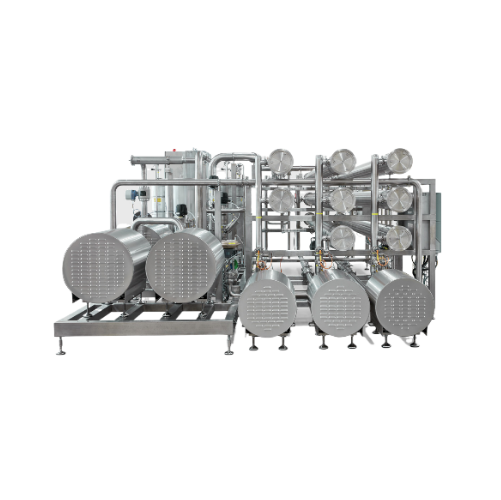
Cip recovery system with membrane filtration
Reduce chemical waste and operating costs by recovering over 90% of spent cau...

Very high pressure homogenizer compression block
Experience reliable performance at extreme pressures with a compression b...

Laboratory homogenizer for scaling nisox-valve benefits
Achieve reliable scaling from lab to production with this precise...

Variable retention time carton freezer
Optimize your production line with a flexible freezing solution that handles multipl...

Aseptic liquid dosing system
Ensure precise, sterile dosing of aseptic liquid products, enhancing product integrity and exte...

Lab-scale spray dryer for small volume powder samples
When developing new formulations, achieving consistent powder quali...

Multiple-effect distillation plants for industrial applications
Optimize your energy use with multiple-effect distillati...
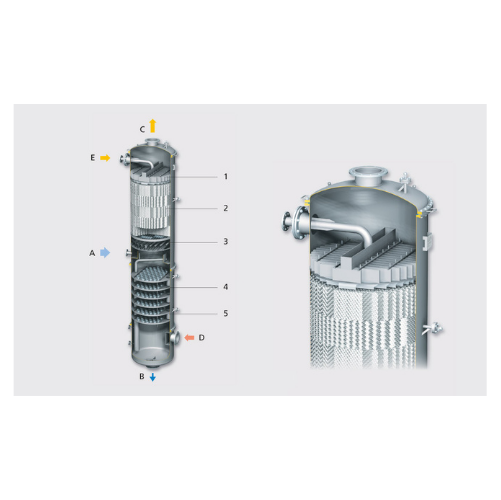
Industrial tray and packing column solutions
For efficient separation and product purification, tray and packing columns o...

Coffee extractor for optimal flavor preservation
Unlock the potential of your extracts with a continuous system that ensur...

Industrial chiller with high part-load efficiency
Optimize your cooling processes with a versatile chiller designed for p...

High-efficiency ammonia chiller package for industrial applications
Optimize your cooling and refrigeration processes w...

Vertical autoclaves for laboratory use
Optimize your lab’s sterilization and drying processes with versatile vertical...

Level bottle filler for wine and spirits
Achieve consistent fill levels with precision sensor technology, ideal for ensurin...

Volumetric bottle filler for distilled spirits
Ensure precise volume control and compliance with regulatory standards in y...

Counter pressure filler for carbonated beverages
Ensure optimal carbonation and minimal oxygen exposure during bottling, e...

Counter pressure can filler for carbonated beverages
Achieve precise carbonation levels and minimal oxygen exposure in ca...

Counter pressure bottle filler for carbonated beverages
Maintain carbonation levels while filling bottles efficiently, en...

Counter pressure bottle filling system for carbonated beverages
Enhance your bottling efficiency with a versatile counte...

Blow/fill/seal packaging system for sterile liquid products
Streamline your liquid product manufacturing with high-speed...
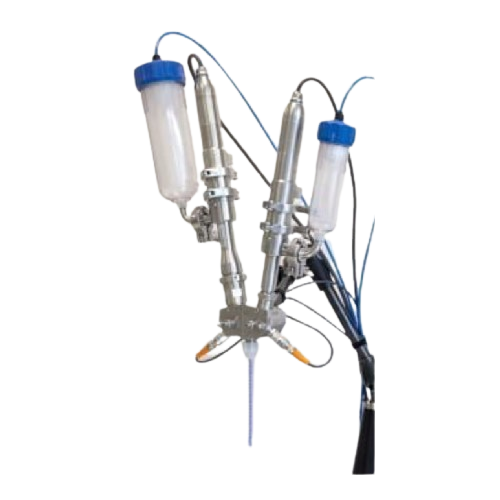
Continuous mixing and filling system for viscous materials
Experience precise, pulsation-free mixing and filling of visc...
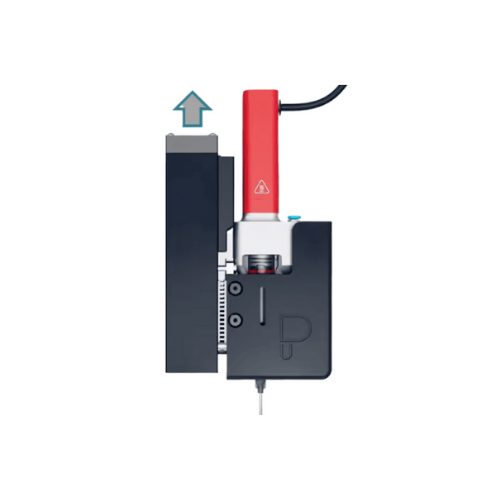
Temperature control system for bioprinting with gelatin
Ensure precise bioprinting outcomes by maintaining accurate tempe...

Rotary pouch packaging system for food and powder products
Streamline your packaging process with a rotary system that e...

High-capacity stick pack packaging solution
Enhance your production efficiency by packaging diverse products like powders ...

Continuous motion liquid packaging system
Achieve precise and clean liquid packaging with advanced servo-driven squeegee s...
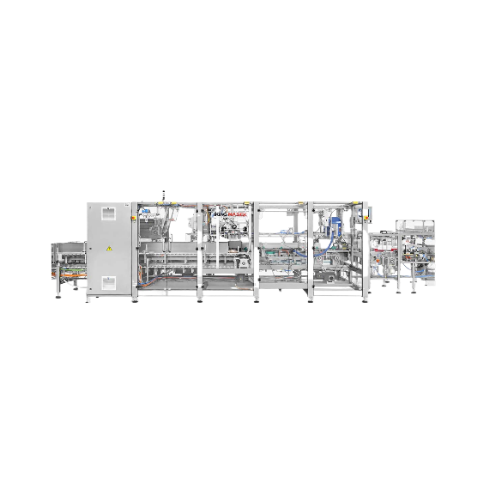
Endload automatic cartoning solution
Streamline your packaging line with this versatile cartoning solution, perfect for eff...
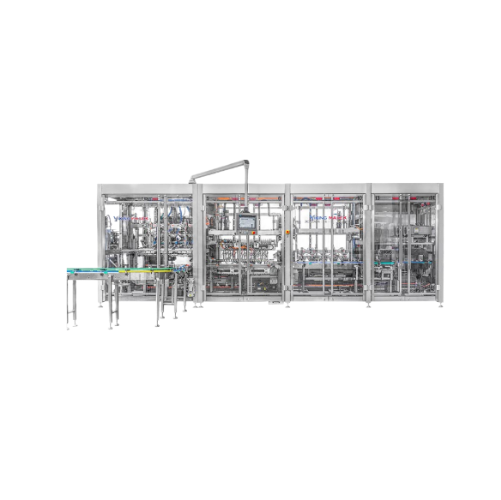
Topload cartoning system for efficient product packaging
Streamline your packaging line with this compact system that com...

Top load cartoner for various carton shapes
Optimize your production line with a versatile cartoning solution, perfect for...

Topload cartoner for packaging cartons
Efficient flap closing for diverse carton sizes, ensuring gentle handling of sensiti...

Rotary premade pouch filler and sealer
Maximize production efficiency by seamlessly filling and sealing diverse pouch sizes...

Automatic rotary filler and sealer for premade pouches
Enhance your production line efficiency with a dual-lane system th...
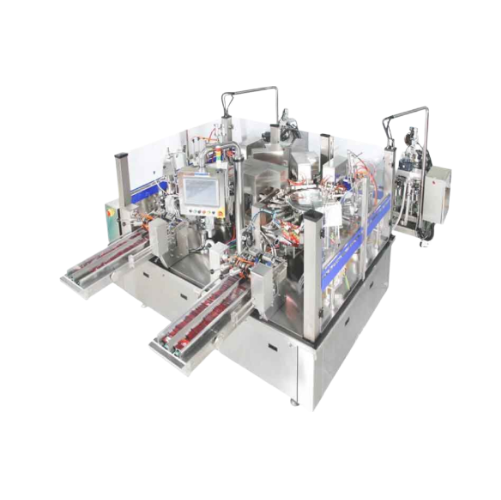
Automatic rotary premade pouch filler and sealer
Optimize your production line with high-speed pouch filling and sealing, ...

Automatic rotary premade pouch filler for various industries
Streamline your pouch packaging process with precision fill...

Automatic rotary premade pouch filler for food products
Enhance your packaging line with a high-speed solution designed t...

Product inspection systems for contamination control
Ensure product safety and compliance with precision inspection techn...

Automated infeed and outfeed systems for packaging lines
Streamline your production line by integrating reliable infeed a...

Support structures for packaging lines
Enhance safety and efficiency in your production line with robust support structures...

Modified atmosphere packaging solutions for perishables
Extend the freshness and shelf life of perishable goods with prec...

Robotic palletizing and depalletizing systems
Enhance your production efficiency by automating the repetitive and labor-in...

Industrial printers and labelers for packaging
Ensure precise package identification and traceability with robust printing...

Industrial product fillers for precise packaging
Achieve consistent package weights and volumes with precision filling sol...

Robotic packaging integration for automated systems
Enhance your production line efficiency with seamless robotics integr...

Hygienic plate heat exchanger
Optimize temperature control for liquid processing with precision-engineered heat exchangers, ...

Automated filter testing for respirator filters and media
Ensure the highest respiratory safety by accurately testing fi...

Automatic inspection system for ampoules and vials
Ensure precision in pharmaceutical production with a comprehensive ins...

Water revitalizer for enhanced hydration and vitality
Transform ordinary water into a structured liquid with enhanced oxy...

Automatic liquid nitrogen storage system for biological samples
Ensure the integrity of your biological samples with a m...

Cell expansion system for antibody drug production
Enhance your biopharmaceutical production with efficient cell expansio...
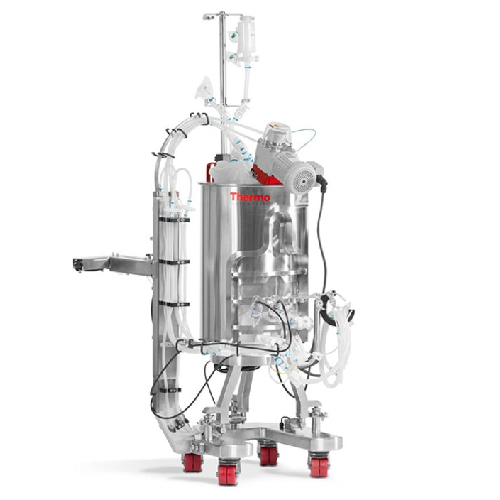
Single-use bioreactor for protein expression
Optimize your biopharmaceutical production with an advanced single-use biorea...

Single-use bioreactor for cell culture processes
Optimize your cell culture productivity with advanced mixing dynamics tha...

Glass bioreactor for mammalian cell culture
Optimize your bioprocessing with advanced mixing and cooling capabilities, des...
Multiplex Rt-qpcr master mix for diagnostic development
Efficiently multiplex up to six targets with high sensitivity, ev...

Benchtop bioreactor for research and development
Achieve precise control over fermentation and cultivation processes with ...

Industrial-scale bioreactors for large-scale biomanufacturing
When producing at large volumes, efficient and reliable bi...
Sublimation front monitoring system for freeze drying
Ensure precise control over your freeze-drying process with real-ti...

Downflow booths for hazardous material processing
Ensure product integrity and operator safety during hazardous material ...

Class Ii biological safety cabinet
Ensure optimal safety levels while handling biological agents with a compact, ergonomic ...

Capsule checkweighing system for pharmaceutical production
Ensure every capsule meets your precision weight standards wi...

Capsule filling for nutrition and health products
Optimize your production line with advanced capsule filling capabilitie...

Capsule filling for pharmaceuticals and nutraceuticals
Enhance your capsule production capabilities with modular filling ...
Robotic pick and place platform for food packaging
Enhance your packaging efficiency and product quality with a scalable ...

Assembly solutions for medical devices
Optimize your medical device production with specialized assembly solutions that ens...

Industrial bag sealing solution for medical and food applications
Enhance your packaging line with reliable and versati...

Mobile biodecontamination unit for cleanroom environments
Achieve rapid, high-capacity sterilization in cleanrooms effor...

Pharmaceutical grade washer for large components
Ensure compliance and maximize productivity with a high-performance solut...

Controlled freezer for biological samples
Achieve precise and reliable freezing without hazardous refrigerants, ensuring t...

Low temperature bath for sample freezing and temperature control
Achieve precise temperature control from -80°C to +100°...

Combi r&d ampoule and vial filling equipment
Optimize your R&D operations with a versatile machine designed for preci...

Medical lab sterilization autoclave
Ensure product sterility with high-performance ventilated autoclaves designed to optimi...

Film shrink wrappers with 90° infeed
Easily adapt to compact spaces while efficiently wrapping various container shapes, fr...

Reverse osmosis water treatment system for medical device cleaning
Ensure purity and compliance by seamlessly integrati...

Portable autoclave for sterilizing surgical instruments
Ensure precision and safety in sterilizing surgical instruments, ...

Automatic washer-disinfector for surgical and dental instruments
Ensure optimal hygiene and performance in medical instr...

Ultrasonic cleaner for surgical instruments
Ensure precise cleaning and disinfection of complex surgical instruments and d...

Vial washing drying filling sealing line for pharmaceutical production
Streamline your vial production with this integr...

Storage cabinet for endoscopes
Ensure the safe, contamination-free storage and easy monitoring of endoscopes with a hanging ...
High energy medical electron linear accelerator for radiotherapy
Optimize cancer treatment precision and efficiency with...

Medical drying cabinet for surgical instruments and glassware
Achieve optimal drying for precision instruments and glass...

Cassette autoclave for sterilization in medical settings
Ensure the reliable sterilization of medical and laboratory inst...
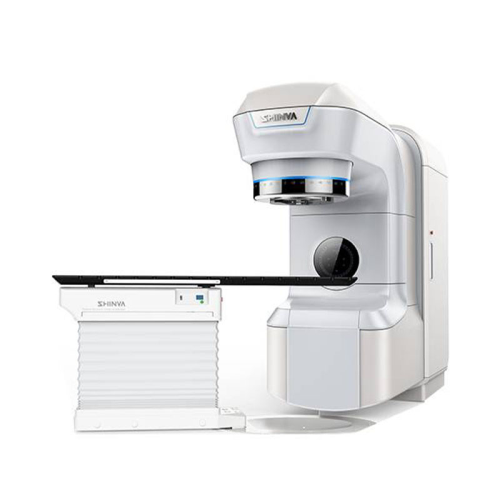
Dual energy medical linear accelerator for radiotherapy
Achieve precise target localization and treatment with advanced d...

Automatic flexible endoscope washer-disinfector
Ensure thorough sanitization of flexible endoscopes with a solution that m...

Class Ii biological safety cabinet for laboratory use
Ensure maximum protection for your laboratory personnel and samples...

Tangential flow filtration system for cell harvesting
Optimize your biopharmaceutical production by integrating a versati...

Rotary transfer system for pharmaceutical product filling
Ensure precise and hygienic filling of diverse pharmaceutical ...

Production system for molecular diagnostics
Eliminate bottlenecks in molecular diagnostics production with a versatile sys...

Filling and assembling solution for Ivd and Poc products
Optimize precision and efficiency in cleanroom environments with...
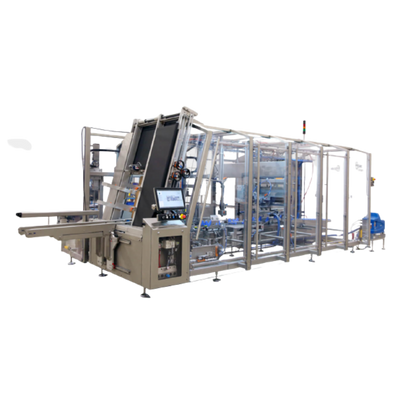
Side Load Case Packer For Facial Tissues
When packing facial tissue boxes into large cases at high speeds, adjustable infee...

Facial Tissue Box Cartoning Machine
Tissues come in various formats, such as men’s sizes, family boxes, or travel tissue pa...