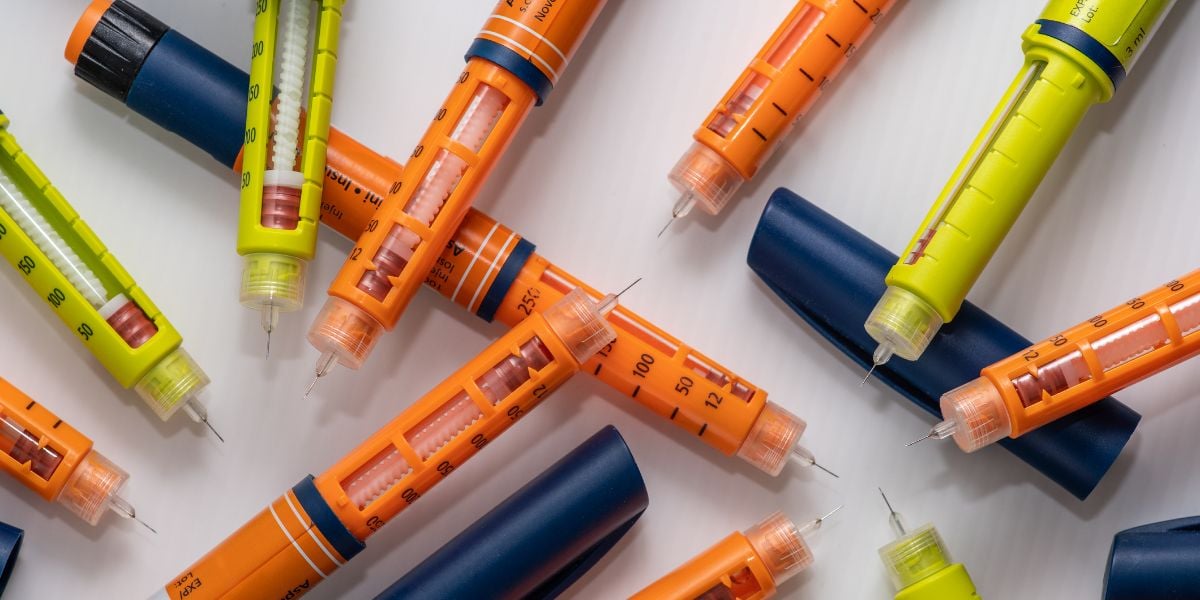
Assembly solutions for medical devices
Optimize your medical device production with specialized assembly solutions that ensure precise handling, labeling, and packaging, streamlining operations for auto-injectors, IV catheters, and more.

Assembles, Labels, and Packages Medical Devices
The Range Device Assembly (RMA) system from Syntegon is a versatile solution for assembling, labeling, and packaging specialized medical devices such as insulin pens, IV catheters, and prefilled syringes. This system excels in flexibility, accommodating both small batch clinical trials and high-speed production lines. It employs modular assembly machines capable of handling complex tasks, from precise component assembly to accurate labeling and efficient packaging. The system supports semi-automatic and fully-automatic operations, integrating seamlessly with upstream and downstream processes, including filling and sealing. With a processing capacity of up to 200 devices per minute, it offers high throughput while maintaining stringent cleanability and control standards. Designed with easy maintenance and CIP capabilities, it complies with GMP standards, making it ideally suited for pharmaceutical and MedTech applications. Its modular design ensures customization options to meet specific production requirements, with engineering support available for tailored solutions.
Benefits
- Maximizes production efficiency with high output rates up to 200 devices per minute.
- Reduces setup time with agile format changeovers and modular design.
- Ensures rapid adaptation to production needs with scalable automation options.
- Supports compliance with GMP standards for safe pharmaceutical manufacturing.
- Integrates effortlessly into existing lines for seamless operation and minimal disruption.
- Applications
- Cannulas, Infusion sets, Pens, Syringes, Vials, Iv catheters, Cartridges, Auto-injectors, Specialized medical devices, Safety devices
- End products
- Intravenous cannulas, Prefilled cartridges for medication, Blood transfusion sets, Drug delivery catheters, Needleless connectors, Epinephrine auto-injectors, Insulin pens, Safety-engineered needles, Dialysis catheters, Prefilled syringes, Vaccine vials, Chemotherapy infusion sets
- Steps before
- Component preparation, Manual loading and unloading, Calibrating processes
- Steps after
- Labeling, Packaging, Tray loading, Visual Inspection, Integrity Checking
- Input ingredients
- auto-injectors, safety devices, pens, components, cannulas, line sets, IV catheters, needles
- Output ingredients
- assembled medical devices, labeled medical devices, packaged medical devices, labeled auto-injectors, labeled pens, assembled infusion sets, assembled catheters, assembled needles, up to 200 catheters per minute, up to 200 devices per minute, up to 120 needles per minute
- Market info
- Syntegon is known for its expertise in processing and packaging technology, offering innovative solutions for the food and pharmaceutical industries. It has a strong reputation for advanced equipment, sustainability, and customer-oriented service.
- Speed
- Up to 200 devices/min
- Capacity
- Device dependent, up to 200 catheters or needles/min
- Size
- Machine dimensions
- Automation
- Manual, Semi-auto, Fully automated
- Output
- Between 1 to 5 devices per minute (manual), Up to 200 devices/min (fully automated)
- Integration
- Fully integrated controls and HMI
- Drive
- Servo and magnetic driven linear units
- Installation
- Compact design for efficient footprint
- Changeover time
- Quick format changeovers
- Marking technology
- Inline laser or other marking technologies
- Automation level
- Manual / Semi-automatic / Fully automatic
- Batch vs. continuous operation
- Batch / Inline Continuous
- Changeover time
- Quick format changeovers
- CIP/SIP
- CIP Compatible / SIP Capable
- Cleaning method
- Easy cleanability
- Energy efficiency
- Efficient footprint
- Output rate
- Up to 200 devices per minute
- Scalability
- Scalable for different production capacities
- Integration capability
- Upstream and downstream integration
- Cleanability
- Easy cleanability
- Corrosive resistance (e.g. acids)
- Yes
- Machine footprint
- From 1m x 1.5m
- Output
- Device dependent
- Capacity
- Depending on device and level of automation
- Drive
- Servo and magnetic driven linear units for precise movements
- Modular design
- Facilitates future upgrades
- Control panel type
- Integrated HMI with full control of all parameters
- Hygienic design
- Easy cleanability and line clearance
- Control panel type
- Integrated HMI
- Integration possibilities
- Upstream and downstream integration
- Modular design
- Flexible platform types
- Scalability
- Manual, semi, and fully automated systems