Continuous manufacturing for pharmaceuticals
Achieve precision in oral solid dosage production with seamless continuous processes that enhance product uniformity and quality while reducing development time and material usage.
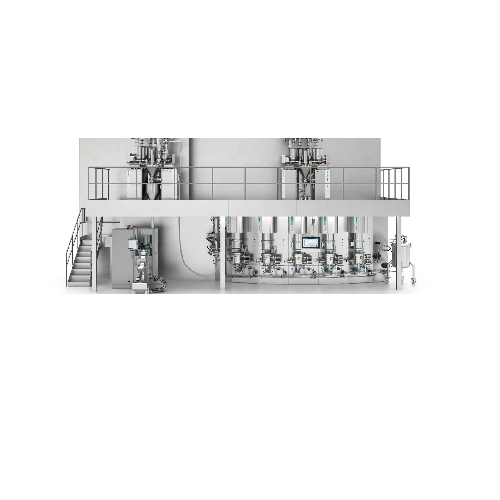
Enables Precise Dosing and Continuous Production
The Xelum Continuous Manufacturing Platform from Syntegon is a transformative solution for the pharmaceutical and nutraceutical industries, offering seamless integration of precise dosing and continuous production for oral solid dosage forms. By leveraging discrete mass dosing, the Xelum platform eliminates the need for traditional scale-up, ensuring high content uniformity even for APIs below 1%. This system is ideal for producing a variety of products such as antibiotic tablets and antiviral capsules, thanks to its robust fluid bed processors that handle granulation and drying in a single chamber, avoiding wet granulate transfer and enhancing reliability. With throughput capacities ranging from 10 to 100 kg/h, Xelum supports rapid production cycles and maintains exact standards in API concentration.
The platform is fully automated, featuring PLC-controlled operations with direct process parameter transfers from R&D to production, allowing for efficient pilot and clinical trials without the constraints of scaling up. It accommodates complex formulations with contained capabilities for OEB4 materials and integrated WIP systems. Xelum’s design prioritizes energy efficiency, reducing production costs while maintaining flexibility for both tablets and capsules. Engineered to meet stringent GMP and FDA standards, it offers a clean-in-place (CIP) system ensuring fast, contamination-free cleaning, perfect for high-potent API development. Customization support allows for tailored configurations to suit specific manufacturing requirements, making Xelum a cornerstone for forward-looking production strategies.
Benefits
- Streamlines R&D to production transfer, eliminating time-consuming scale-up.
- Ensures high content uniformity in low API concentrations, enhancing product quality.
- Minimizes operational costs with reduced energy consumption and efficient process integration.
- Offers high flexibility with seamless transition between tablet and capsule production.
- Promotes regulatory compliance with GMP and FDA standards for safe manufacturing.
- Applications
- Nutraceuticals, Granules, Pellets, Oral solid dosage forms, Tablets, Capsules, Pharmaceuticals
- End products
- Multivitamin pellets, Pain relief capsules, Energy supplement tablets, Calcium supplement granules, Antibiotic tablets, Iron supplement capsules, Probiotic tablets, Antiviral capsules, Chemotherapy granules, Antifungal granules
- Steps before
- API dosing, Blending, Encapsulation preparation, Granulation preparation
- Steps after
- Tablet pressing, Capsule filling, Inspection, Packaging
- Input ingredients
- active pharmaceutical ingredients (APIs), excipients, raw materials, powder, granules
- Output ingredients
- granules, tablets, capsules, sachets, mini tablets
- Market info
- Syntegon is known for its expertise in processing and packaging technology, offering innovative solutions for the food and pharmaceutical industries. It has a strong reputation for advanced equipment, sustainability, and customer-oriented service.
- Accuracy
- Precisely dosed less than 1% API
- Automation
- Automated DoE support
- Batch Size
- Starting from 250 g
- Throughput
- 10 kg/h to 50-100 kg/h
- Continuous Granulation
- Up to 30 kg/h
- API Concentration Precision
- Down to 0.25%
- Containment Capabilities
- OEB4 with WIP
- Scalability
- Direct 1
- Granulation Yields
- High granulation yields
- Particle Size Distribution
- Unimodal distribution
- Cleaning Process
- Automated cleaning in one shift
- Blending and Granulation
- Same machine for both processes
- Compression and Encapsulation
- Seamless continuous process
- Content Uniformity
- High content uniformity
- Product Quality Control
- Easy and reliable quality control
- Traceability
- Integrated traceability
- Footprint Reduction
- Up to 50% reduction
- Working Mechanism
- Continuous direct compression and encapsulation
- Integrated Steps
- Dosing, mixing, granulation
- Automation Level
- PLC-controlled with automated DoE support
- Batch vs. Continuous Operation
- Continuous
- CIP/SIP
- Integrated WIP (washing in place)
- Energy Efficiency
- Lower due to minimized losses
- Changeover Time
- Direct transfer from R, D to production without scale-up
- Density/particle size
- 0.5–2.5 g/cm³ / 50–1000 µm
- Control panel type
- Automated / User-friendly interface
- Discharge method
- Continuous / Intermittent
- Control panel type
- HMI touch screen
- Integration possibilities
- PLC / SCADA integration
- Discharge method
- Continuous / Direct
- Compact footprint
- Yes
- Containment capabilities
- OEB4 with WIP