Frozen storage and shipping for biopharmaceuticals
Ensure the integrity of critical biopharmaceuticals with a robust solution for secure frozen storage and reliable shipping, ideal for protecting valuable therapeutics through demanding freeze and thaw cycles.

Preserves and Transports Biopharmaceuticals Securely
The Frozen Storage and Shipping System from Sartorius is engineered to preserve and transport sensitive biopharmaceuticals, ensuring product integrity across the cold chain. Designed for applications from monoclonal antibodies to mRNA-based therapeutics, these systems use advanced freeze/thaw technology to protect product quality during storage and shipping. The systems feature a range of configurations, including scalable lab to commercial-scale solutions, supporting flexible operations. Integrated fluid management ensures seamless processing, while the stainless steel construction provides excellent corrosion resistance. Compliance with industry standards like GMP and FDA affirms suitability for pharmaceutical manufacturing. The equipment includes customizable options and comprehensive engineering support to meet specific processing needs.
Benefits
- Ensures product integrity during transport with robust freeze-thaw cycles.
- Streamlines operations from lab to industrial scale, accommodating growth.
- Minimizes risk of contamination with automated fluid management.
- Facilitates regulatory compliance with GMP and FDA standards.
- Offers flexible, scalable solutions to match production demands.
- Applications
- Vaccines, Cell therapy, Mrna production, Biosimilars, Biopharmaceuticals, Gene therapy, Blood and plasma
- End products
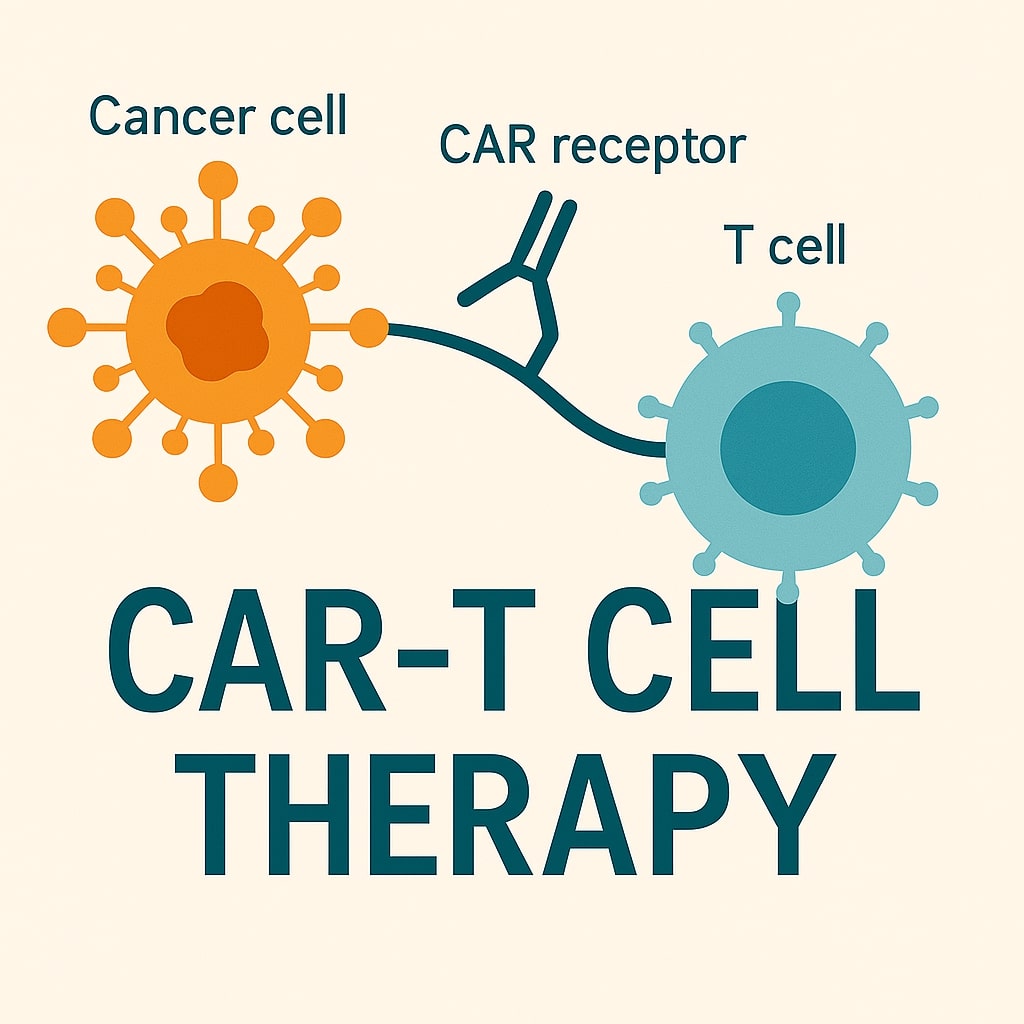
- Car-t cell therapies, Plasma-derived clotting factor, Rituximab biosimilar, Mrna-based therapeutics, Monoclonal antibodies, Covid-19 vaccines, Allogeneic stem cell therapies
- Steps before
- Bioreactor Culture, Fermentation, Cell Culture Harvesting
- Steps after
- Thawing, Cold Chain Distribution, Final Drug Product Formulation
- Input ingredients
- bulk drug substance, sensitive biological products
- Output ingredients
- frozen drug substance, thawed drug substance
- Market info
- Sartorius AG is renowned for manufacturing high-precision laboratory and bioprocess equipment. They specialize in filtration, fermentation, and cell cultivation systems, known for their innovation and reliability in the pharmaceutical and biotech industries.
- Capacity
- 900, 000 L/year
- Automation
- PLC-controlled
- Temperature control
- Constant throughout lifecycle
- Freeze/thaw performance
- Consistent and scalable
- Batch vs. continuous operation
- Batch / Inline Continuous
- CIP/SIP
- CIP 121°C / SIP 135°C
- Cleaning method
- CIP / Manual
- Energy efficiency
- 0.5–2 kWh/kg moisture
- Freeze/Thaw process control
- Automated Temperature Monitoring
- Shipping integrity
- Ensured by global network
- Density/particle size
- 0.5–2.5 g/cm³ / 50–1000 µm
- Temperature Sensitivity
- Ultra-low temperatures
- Container Type
- Cryogenic containers
- Control panel type
- HMI / Touchscreen
- Integration possibilities
- SCADA / PLC / IoT
- Discharge method
- Automatic / Manual
- Compact footprint
- Modular / Small-scale
- Customization level
- Fully customizable
- Container adaptability
- Various sizes
- Temperature range modification
- -20°C to -80°C