Automated sample preparation software
Enhance your laboratory’s efficiency with a software solution that automates sample preparation, ensuring consistent and reliable analysis while seamlessly integrating with existing data management systems. This tool is vital for labs seeking to streamline operations and maintain data integrity across complex workflows.
Automates and Integrates Sample Preparation Processes
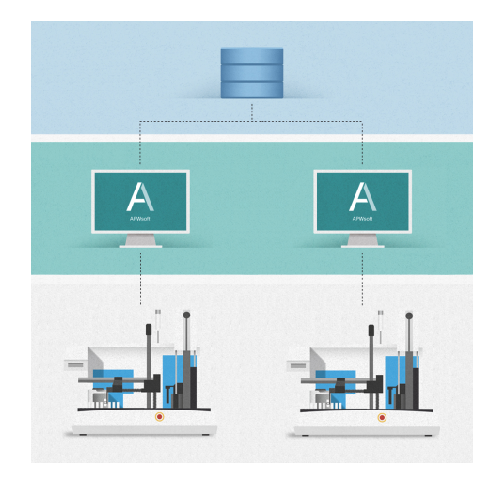
APWsoft by SOTAX is an automated sample preparation software designed to enhance laboratory efficiency and compliance in pharmaceutical and biotech environments. It integrates seamlessly into existing lab workflows, automating data capture, storage, and reporting processes. This software supports a comprehensive range of applications, from solid dosage forms like tablets and capsules to complex formulations like liposomal nanocarriers and injectable suspensions. APWsoft’s intuitive interface and network capabilities facilitate easy method development and transfer across multiple sites, meeting stringent 21 CFR Part 11 compliance standards. It connects directly with analytical instruments like HPLC systems for both online and offline sample management, ensuring precise and reliable data handling. This integration minimizes human error while improving throughput and consistency, essential for high-stakes production lines in pharmaceutical R&D and quality control labs.
Benefits
- Enhances efficiency by automating complex sample workflows, reducing manual handling errors.
- Ensures regulatory compliance with robust 21 CFR Part 11 alignment.
- Increases data reliability by integrating directly with HPLC and CDS systems.
- Facilitates method transfer across multiple sites, supporting consistent production quality.
- Optimizes laboratory productivity through streamlined data management and reporting.
- Applications
- Medical devices, Gels, Pellets, Granules, Microspheres, Catalysts, Stents, Food, Nano suspensions, Fine chemicals, Coated lenses, Soft-gelatin capsules, Implants, Animal health, Capsules, Powders, Washtabs, Transdermal patches, Bottles with screwcaps, Creams, Suppositories, Semi-solids, Apis, Tablets, Injectable suspensions
- End products
- Active pharmaceutical ingredients (ibuprofen, Zeolite catalysts, Paracetamol), Enteric-coated capsules, Anti-inflammatory creams, Eye dropper bottles with child-resistant caps, Orthopedic implants, Microsphere drug delivery systems, Probiotic capsules for animal health, Liposomal nanocarriers, Dishwasher tablets, Omega-3 soft-gel capsules, Anti-reflective coated lenses, Fluocinolone acetonide patches, Rectal suppositories, Hydrogels, Fine chemical intermediates, Talcum powder, Pelleted feed supplements, Cardiac stents, Analgesic tablets, Nutritional protein powders, Insulin suspensions, Pharmaceutical granules
- Steps before
- Sample collection, Measurement of sample weight, Method development
- Steps after
- HPLC analysis, Data reporting, Quality control verification
- Input ingredients
- tablets, capsules, pellets, APIs, powders, granules, soft-gelatin capsules, suppositories, medical devices, stents, implants, microspheres, nano suspensions, injectable suspensions, semi-solids, gels, creams, transdermal patches, washtabs, fine chemicals, catalysts, food, animal health, coated lenses, bottles with screwcaps
- Output ingredients
- sample information, ID, weight, sample concentration, test data
- Market info
- Sotax AG is renowned for its expertise in manufacturing high-quality pharmaceutical testing equipment, including dissolution testing systems, and has a strong reputation for reliability, precision, and innovation in laboratory and industrial applications.
- Automation
- 100% Scalable
- Database
- MS-SQL
- Compliance
- 21 CFR part 11
- Audit Trail
- Readable
- Software Architecture
- Intuitive
- Data Integration
- EmpowerLink™
- Sample Integration
- Online/Offline
- Sample Connectivity
- HPLC / Waters Acquity H-Class+
- Developing Speed
- Advanced Developer Options
- Automation level
- Fully automated
- Method transfer
- Across sites
- Integration capability
- HPLC/Waters Empower CDS
- Data capture and storage
- Central MS-SQL database
- Batch vs. continuous operation
- Batch
- User interface
- Intuitive
- Sample set creation
- Automatic
- Audit trail
- Readable
- Density/particle size
- 0.5–2.5 g/cm³ / 50–1000 µm
- 21 CFR Part 11 Compliance
- Yes
- FDA Compliance
- Yes
- GMP Compliance
- Yes
- CE Marking
- Yes
- Machine footprint
- Compact for laboratory use
- Control panel type
- Touchscreen interface
- Discharge method
- Automated sample ejection
- Control panel type
- Intuitive Software Interface
- Integration possibilities
- HPLC, Waters Acquity, Empower CDS
- Software Platform
- APWsoft, EmpowerLink
- Database compatibility
- MS-SQL
- Automation level
- Fully Automated
- Scalability
- 100% Scalable Software Architecture
- Audit Trail
- Readable Audit Trail
- Compliance
- 21 CFR Part 11
- Central Operations
- Single PC/Networked Operations