Dissolution testing software for automated analysis
Streamline your laboratory testing with seamless data capture and in-depth analysis, ensuring compliance while integrating automated solutions into your research and quality control workflows.
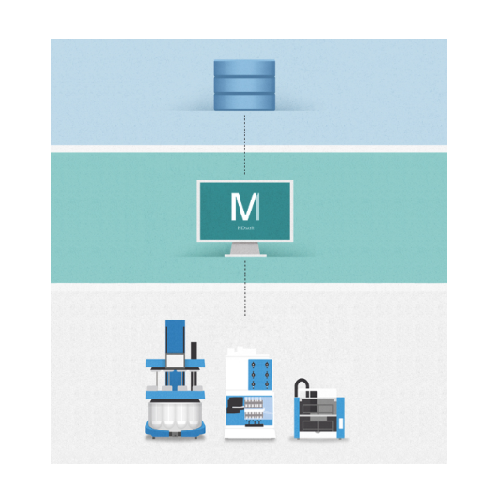
Automates Dissolution Testing and Data Management
MDsoft from SOTAX is a comprehensive dissolution data management system tailored for the pharmaceutical and biotech industries. It uniquely automates the entire process of dissolution testing, from data capture and analysis to reporting, within a fully compliant 21 CFR part 11 environment. MDsoft is integral to the quality control and R&D phases, where precise testing and sampling of products such as pain relief tablets, vitamin capsules, and injectable suspensions are critical. The software supports scalability across single or networked PCs and integrates seamlessly into existing IT infrastructure, enabling streamlined data handling with a central SQL database. Its innovative features, like integrated video monitoring, allow for detailed R&D visualization and QC troubleshooting. MDsoft also facilitates compliance with key industry standards and reduces manual intervention with its automation capabilities, enhancing productivity and data integrity.
Benefits
- Enhances data integrity and compliance with 21 CFR part 11 standards.
- Improves productivity by automating dissolution processes and data management.
- Allows precise R&D and QC insights through integrated video monitoring.
- Facilitates seamless integration into existing network infrastructures.
- Streamlines reporting and data export to ELN/LIMS, reducing manual effort.
- Applications
- Medical devices, Gels, Pellets, Granules, Microspheres, Catalysts, Stents, Food, Nano suspensions, Fine chemicals, Animal health products, Coated lenses, Soft-gelatin capsules, Implants, Api's, Capsules, Powders, Washtabs, Transdermal patches, Creams, Suppositories, Semi-solids, Bottles with screw caps, Tablets, Injectable suspensions
- End products
- Zeolite catalysts, Orthopedic implants, Insulin injectable suspensions, Herbal supplement pellets, Hydrocortisone creams, Liposomal nano suspensions, Glycerin suppositories, Pet health nutritional products, Amino acid food supplements, Microsphere drug carriers, Omega-3 soft-gel capsules, Anti-reflective coated lenses, Protein powders, Active pharmaceutical ingredients (apis) for antibiotics, Effervescent granules, Dishwasher washtabs, Nicotine transdermal patches, Pain relief tablets, Anti-aging gels, Coronary stents, Vitamin capsules, Refillable water bottles with screw caps
- Steps before
- Sample Preparation, Media Preparation, Tablet Formulation, Capsule Filling
- Steps after
- Data Management, Report Generation, Video Monitoring Analysis, Compliance Verification, Advanced MCA Evaluation, Data Export to ELN/LIMS
- Input ingredients
- tablets, capsules, pellets, active pharmaceutical ingredients (API's), powders, granules, soft-gelatin capsules, suppositories, medical devices, stents, implants, microspheres, nano suspensions, injectable suspensions, semi-solids, gels, creams, transdermal patches, washtabs, fine chemicals, catalysts, food, animal health products, coated lenses, bottles with screwcaps
- Output ingredients
- dissolution data, video footage, multi-component analysis results, compliance reports, customized reports, product quality analysis
- Market info
- Sotax AG is renowned for its expertise in manufacturing high-quality pharmaceutical testing equipment, including dissolution testing systems, and has a strong reputation for reliability, precision, and innovation in laboratory and industrial applications.
- Automation
- Full
- Compliance
- 21 CFR part 11
- Integration
- Video monitoring
- Operating System Compatibility
- Windows 10
- Database
- MySQL
- Components Evaluation
- Up to 5
- Software Architecture
- Scalable
- Automation level
- Fully automated
- Batch vs. continuous operation
- Batch
- Cleaning method
- CIP / Manual
- Compliance compatibility
- 21 CFR part 11 compliant
- Data export options
- ELN/LIMS compatible
- Video monitoring integration
- Built-in video monitoring
- Network capability
- Standalone / Networked operation
- Report configuration
- Customizable reports
- Scalability
- 100% scalable software architecture
- Security management
- User-group configuration
- Density/particle size
- 0.5–2.5 g/cm³ / 50–1000 µm
- Machine footprint
- Compact
- Camera Mounting System
- Adjustable
- Camera Lighting
- Indirect lighting source
- Camera Space
- Protected central space
- Control panel type
- Centralized
- Software Architecture
- Scalable software architecture with central MySQL database
- Control panel type
- Touchscreen / HMI
- Integration possibilities
- ELN/LIMS compatibility
- Software scalability
- 100% scalable architecture
- Video monitoring
- Integrated video monitoring with adjustable cameras
- Central database
- MySQL