Mo-99 dispensing and packaging hot cell
Safely manage high-activity radiopharmaceuticals with this advanced hot cell, designed for precise Mo-99 handling and seamless integration into nuclear medicine operations.
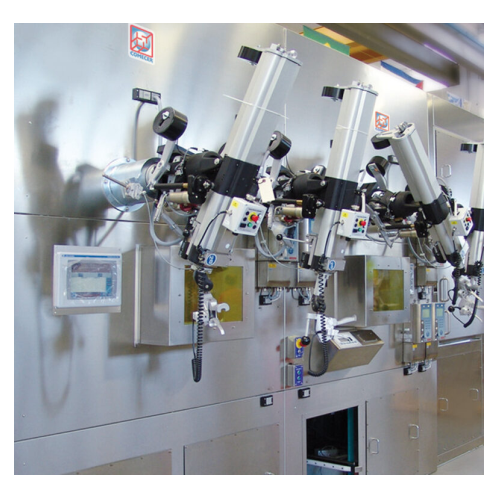
Dispenses, Packages, and Stores Mo-99
The Mo-99 Dispensing and Packaging Hot Cell System from Comecer is a specialized solution designed for the safe and efficient processing of radiopharmaceuticals. This system excels in the precise dispensing, packaging, and storage of Mo-99, tailored for nuclear medicine and radiopharmaceutical manufacturing. It boasts a cGMP-compliant double hot cell setup, providing a robust environment for handling up to 65 TBq of Mo-99 and offering extreme radiation protection with 180mm lead shielding.
The system’s working principle involves fractionation, calibration, and preparation of Mo-99 aliquots, suitable for producing multi-dose vials and Technetium-99m generators. With automation systems for the containment handling of B(U) transport containers and SIL-3-certified safety interlocks, it ensures operational safety and process integrity.
This equipment integrates seamlessly into your production line, with specialized calibration systems and waste extraction features, and supports batch operations. It includes options for manual or automated handling, with customization possibilities to adapt to various transport formats. It complies with IEC 61508 standards, providing peace of mind for stringent regulatory environments.
Benefits
- Enhances safety and operational integrity with SIL-3-certified safety interlocks.
- Ensures exceptional radiation protection with 180mm lead shielding.
- Increases efficiency in radiopharmaceutical production through seamless integration with existing lines.
- Reduces regulatory compliance stress with cGMP-compliant design.
- Optimizes resource use by supporting both manual and automated batch operations.
- Applications
- Active pharmaceutical ingredients (apis), Nuclear medicine, Radiopharmaceuticals
- End products
- Mo-99 aliquots, Iodine-131 capsules, Multi-dose mo-99 vials, Technetium-99m generators
- Steps before
- Fractionation, Calibration, Preparation for shipment
- Steps after
- Packaging, Storage, Dose Calibration
- Input ingredients
- Mo-99 bulk, 65 TBq 99Mo, B(U) transport containers
- Output ingredients
- Mo-99 aliquots, multi-dose vials, packaged containers, waste
- Market info
- Comecer is known for manufacturing advanced containment and isolation systems, particularly in the pharmaceutical and nuclear medicine sectors, with expertise in custom-engineered equipment, ensuring high safety and compliance standards.
- Number of working chambers
- 3 + 2
- Type of process
- Non sterile API manufacturing
- Type of shielding
- Lead 180mm / Lead 150mm
- Automation systems available
- Containment handling of B(U) transport containers
- Productivity
- Up to 20 multi-dose / batch vials
- SIL Rating
- SIL-3 certified safety interlocks
- Handling capacity
- Up to 65 TBq 99Mo
- SIL-3 Certified Safety Interlocks
- SIL-3 (IEC 61508)
- Automation level
- PLC/SCADA
- Batch vs. continuous operation
- Batch
- Handling capacity
- Up to 65 TBq 99Mo
- Waste extraction system
- Included
- Transport flask connection
- 1.2 Tons
- Cleanability
- Easy to clean hot cell surface
- Hot Cell Type
- Double
- GMP Compliance
- cGMP
- IEC Safety Standard
- SIL-3 ref IEC 61508
- Shielding thickness
- Lead 180mm / Lead 150mm
- Number of working chambers
- 3 + 2
- Transport flask connection
- 1.2 Tons capacity
- Control panel type
- PLC / HMI
- Integration possibilities
- Automated containment handling systems
- Safety systems
- SIL-3 certified
- Shielding type
- Lead 180mm / Lead 150mm