Industrial filter dryer for high potent Api production
Optimize your process of producing high-potency pharmaceuticals by efficiently filtering, washing, and drying active ingredients with maximum containment and precision.
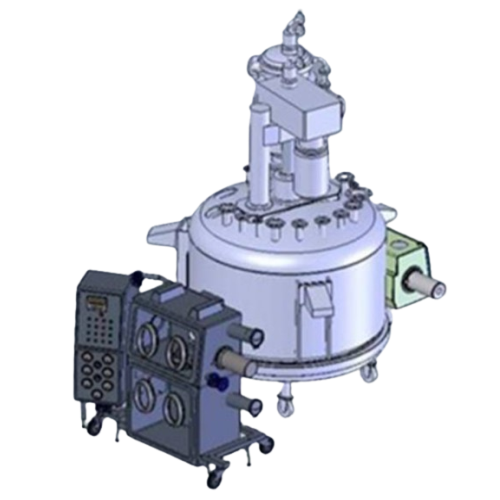
Filters, Washes, and Dries High-Potency API Slurries
The Filter Dryer RGFD 3.0 – 2.4602 from Rosenmund, part of De Dietrich Process Systems, is engineered for precision handling of high-potency pharmaceuticals in the API production line. It expertly filters, washes, and dries API slurries, ensuring optimal product purity and consistency. This equipment integrates seamlessly into pharmaceutical and fine chemical processes, accommodating batch operations with manual control for versatility. Its unique design includes a double chamber isolator, enhancing contamination protection during product discharge. Built with advanced vacuum drying technology, the RGFD 3.0 maximizes energy efficiency and reduces drying times. Compliance with GMP standards ensures it meets rigorous regulatory requirements, making it an ideal solution for manufacturers producing cytostatic tablets and chemotherapy agents. The equipment supports integration with existing facilities, providing CAD models to fit seamlessly into your production layout. Constructed from materials compatible with high-corrosion environments, it guarantees durability and long service life. Maintenance is simplified with accessible parts and options for in-place cleaning systems, minimizing downtime and ensuring consistent operational efficiency.
Benefits
- Enhances product purity and safety by minimizing cross-contamination with isolator systems.
- Reduces energy usage and drying times due to vacuum drying technology.
- Complies with GMP standards for seamless regulatory approval in pharmaceutical production.
- Durable construction suitable for high-corrosion environments, ensuring longevity and reliability.
- Simplifies integration and reduces installation costs with customizable CAD layout support.
- Applications
- Cytostatic drugs, Active pharmaceutical ingredients (api), Fine chemicals, Pharmaceuticals
- End products
- Cytostatic tablets, Chemotherapy agents, High-potency pharmaceuticals, Cancer treatment capsules, Anticancer drugs
- Steps before
- Purification, Slurry Preparation, API Production, Emulsification
- Steps after
- Sampling, Sterilization, Packing, Coating
- Input ingredients
- API, mother liquor, slurry
- Output ingredients
- high potent cytostatic drug, filtered product, washed product, dried product
- Market info
- De Dietrich is renowned for manufacturing high-quality engineered-to-order (ETO) equipment, specializing in chemical process systems, glass-lined reactors, and filtration technologies, with a strong reputation for innovation, reliability, and safety in the chemical and pharmaceutical industries.
- Filtration
- Filtration, Washing, and Drying
- Discharge Method
- Double chamber isolator with additional pusher port isolator for heel recovery and sampling
- Compatibility with 3D Modeling
- 3D Model layout provided with proposal submission
- Working mechanism
- Filtration, Washing, Drying
- Batch vs. continuous operation
- Batch
- Discharge method
- Double chamber isolator with pusher port
- Automation level
- Not specified
- CIP/SIP
- Not specified
- Changeover time
- Not specified
- Energy efficiency
- Not specified
- Cleaning method
- Not specified
- Container size and shape
- Adaptable to various container types and sizes
- Machine footprint
- N/A
- Basket shape and size
- N/A
- Cone shape and size
- N/A
- Tank size
- N/A
- Feed method
- Slurry of API and mother liquor
- Discharge method
- Double chamber isolator
- Isolation ports
- Additional pusher port isolator
- Discharge method
- Double chamber isolator with additional pusher port isolator
- 3D Model layout
- Provided with proposal submission