
Dry powder inhalants (dpi)
Find innovative production technology for making dry powder inhalants (dpi) and connect directly with world-leading specialists
Let's navigate the complex world of engineered-to-order production technology together. Whether you're building a new process or optimizing an existing line, our platform connects you directly to the experts who can help. Use our curated catalogue to dive straight into the technologies that match your production goals. We continuously map out how production technology from suppliers around the world can help solve real production challenges. If you find something interesting, we introduce you directly to the specialists who know how to implement it. With more than 600 trusted machine manufacturers and over 20.000 technical experts in our network, you’re never far from the answers you need.
Tell us about your production challenge
Processing steps involved in dry powder inhalants (dpi) making
Which dry powder inhalants (dpi) technology do you need?

Moisture and pressure headspace analysis for sealed parenteral containers
Ensure accurate moisture and pressure assess...

Water activity headspace analyzer for drug product samples
Ensure precise control of moisture levels across the entire p...

Table-top capsule filler for micro-dosing
Achieve precise micro-dosing for pharmaceutical powders and pellets with a user-...

Industrial powder grinder
Achieve precise particle size reduction and classification with advanced equipment designed for co...

High shear impact mixer for agglomeration and dispersion
Achieve precise homogeneity and efficient agglomeration with a h...

Super high shear mixer for nano to micron powders
Achieve high-precision mixing and surface treatment of nano to micron-s...

Choppers and disintegrators for industrial size reduction
Enhance your production efficiency by mastering size reduction...

Compactors and granulators for powdery products
Transform loose powders into dense, free-flowing granules that enhance han...

Drying systems for powders and bulk solids
Enhance your production line with precise control of moisture content in powder...

Lab mixer for powder formulations
Achieve precise particle mixing and coating with this stand-alone high-shear lab mixer, d...

Powder characteristic evaluation
Ensure precise powder analysis and testing in your laboratory to optimize production qualit...

Laboratory vacuum dryer for heat-sensitive materials
Optimize moisture control in heat-sensitive materials with precise a...

Cip/sip cleaning for solids processing systems
Ensure seamless transitions and maintain hygienic production environments w...

Containment solutions for hazardous material processing
Ensure safe and efficient processing of hazardous materials with ...
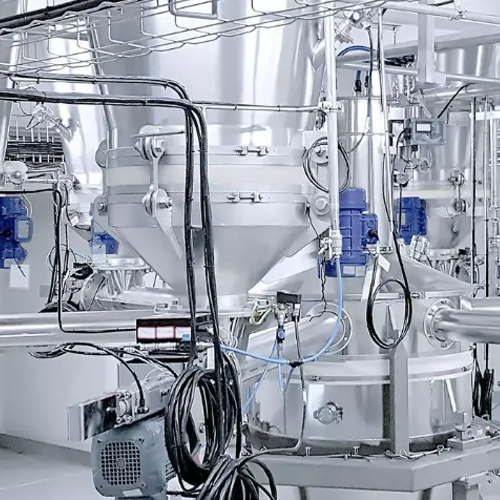
Conical screw mixer for powder blending
Achieve precise and homogeneous blending with the conical screw mixer, ensuring uni...

Ultra-fine powder flash drying system
Achieve rapid moisture removal and particle refinement with this integrated system, d...
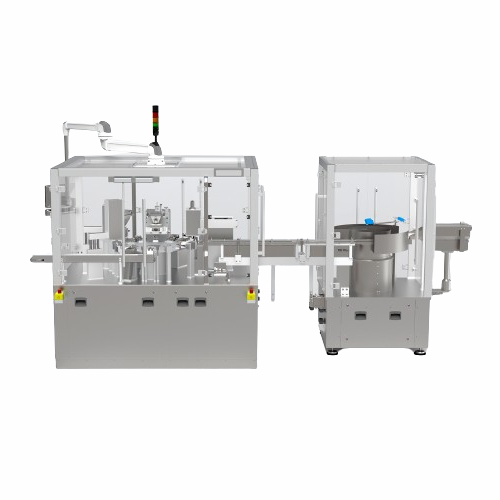
Rotary indexing table for dosing and assembly
Optimize production efficiency with a compact rotary indexing table that str...

Dpi blister strip weighing system
Ensure precision in your inhalation product lines with a system designed for real-time we...

Auger dosing unit for powder dosing in pharmaceuticals
Ensure precise and flexible dosing of pharmaceutical powders with ...

Dpi inhaler production system
Enhance your inhaler production with precision assembly and integrated dosing, filling, and ca...

Commercial blister filling solution for dry powder
Efficiently fill and seal blister packs with precision, ensuring consi...

Micro-dosing system for inhalation blisters
Streamline your inhalation product production with precision micro-dosing, for...

Disc filler for dry powder inhalers
Optimize your dry powder inhaler production with precision micro-dosing for diverse pow...

Custom automation for drug-device filling and assembly
Efficiently integrate precise filling and assembly for biopharmace...

Dry powder filling isolator for cgmp production
Achieve precise powder dosage and secure containment for hazardous materia...

Aseptic spray dryer for pharmaceutical products
Optimize your sterile powder production with precise control over particle...

Laboratory dedusting systems
Designed for high-containment lab environments, this solution efficiently captures and manages ...

Flow controller for Dpi testing
Ensure precise flow regulation and reliable testing for inhaler devices, enhancing accuracy ...

3D powder mixer for active pharmaceutical ingredients
Proper mixing and homogenization are essential to achieve a reliabl...

Modular assembly line for healthcare products
Assembly setups need to be constantly rethought and reconfigured to adapt qu...

High containment big bag discharge docking system
The discharge of materials demands strict containment, particularly in ...
Accurate multiple dosing system for powders
Dosing multiple powders in a batch process is time inefficient, especially whe...
Hygienic floor scale
The food and pharmaceutical industries require maintaining high levels of sanitations as well as logisti...

Hygienic mobile scale
Having a reliable and accurate weighing and measuring solution is absolutely essential, especially in t...

Fast gravimetric powder microdosing 50 - 200 g
Micro-dosing powders of high-value food or chemical products can be challen...

Gravimetric powder microdosing 2 - 100 g
Powders and granules are commonly dosed for the manufacturing of pharmaceuticals, ...

Gravimetric powder microdosing 0.400 - 2 g
If you need to process high value food, pharmaceuticals or chemicals, there is ...

Single-use powder handling bag
Containers for handling pharmaceutical and biopharmaceutical powders may be disposable, to av...

API powders storage bottle
Users who prefer rigid storage over single-use pharma charge bags, require lightweight solutions ...

Single-use containment valve
Safe transfer of liquids and hazardous powders into systems can be a challenge in pharma, bioph...

High containment closing system
Pharma and biopharma laboratories need fast effective solutions for tightly sealing bags for...

High containment sampler
Drawing a sample for evaluation from a running process is not a simple task, especially when dealing...

Metal detector for pharmaceutical powders
Powdered and granulated pharmaceuticals require careful screening against metal ...

R&D V-type powder blender
Many pharmaceutical processes rely on the complete blending and homogenizing of different powdered...

Pharmaceutical container lifter
Moving larger or heavier containers from height is out of the scope for standard handling eq...

Vacuum sack lifter
In the production of pharmaceuticals and other high-hygiene environments, manual handling of inputs or int...

Bin blender
Many pharmaceutical processes require the blending of powders or other granular ingredients, the mixing of settled...

Container lifter
Container handling in the pharmaceutical industry brings its own set of unique challenges. Traditional barrel...

Mobile drum lifter
The production of pharmaceuticals and other hygienic products often requires the handling of large and bul...

Powder transfer vessel
Intermediate bulk storage of pharmaceutical product requires specialist containers. For increased effi...

GMP Jib crane
Vacuum manual handling systems are the ideal way of quickly and safely moving small to medium sized loads in all...

Small feeder with flexible wall hopper
In many laboratory applications and production processes, smaller quantities of powd...

Metal detection system for aluminum packages
The magneto reflection system is ideal for detection of metals in aluminium f...

Checkweigher for sachets and sticks
The checkweighers for multiple lanes have a 1.5 times faster response speed and 2 times...

Fluid bed dryer for production scale
Fluidized bed drying (FBD) is a common process in the pharmaceutical industry for dryi...

Fluid bed dryer for lab scale
Fluidised bed drying (FBD) is a common process in the pharmaceutical industry for drying compo...

Fluid bed dryer and mixer for lab scale
Designed for pharmaceutical R&D, a lab-scale fluid bed dryer and mixer/granulat...

Magnetic agitator
Reliable mixing and agitation are essential to any quality production process that involves a fluid. This n...

Centrifugal disc filter
The pharmaceutical and bioprocess industries need filtration processes of particularly high quality t...

Spherical dryer
Drying vessels that are simple, reliable, and easy to maintain increases product quality and process efficienc...

Filter dryer
The pharmaceutical and bioprocess industries need filtration processes of particularly high quality to ensure the...

Transfer system for high-containment environments
A high-performance transfer port system that combines ultimate safety, ...

Half-suit tester
Designed to allow operators to perform sterility testing in an aseptic environment providing assurance of mat...
Glove tester
Testing device of the containment capability of gloves, which is as important as the other parts of the integrate...
Rotary vacuum dryer
Powdered products tend to agglomerate during vacuum drying. This adds an additional step to the productio...

Premium vacuum conveyor
When you have a need to tailor make your conveyor and still have the high requirement on hygiene, e.g...

Horizontal vacuum dryer with eccentric agitator
Conventional dryers are inefficient and can lead to significant mechanical...

Vacuum tray dryer with clean-in-place system
Cabinet tray dryers can be difficult to clean, making their use difficult for...

Horizontal paddle vacuum dryer
Agitated vacuum dryers can be difficult to clean which makes them unsuitable for multi-produc...

Laboratory-scale vacuum tray drying oven
Vacuum drying is used to remove moisture from sensitive materials. Drying small ba...

Bellows for pharmaceutical powders
Thermal and mechanical changes can lead to stresses in industrial piping systems. These ...
High containment segment ball valve
Valves fitted in blenders and dryers can have dead spaces that prevent complete process...

Removable GMP compliant connections for tubing
Tubing frameworks can have rough welding joints, corners and sharp angles t...

Negative pressure isolator
Used for products biologically hazardous, that also require minimized cross contamination.

Vertical laminar flow cabinet
HEPA filtered vertical laminar airflow (down flow) creates an ISO 14644-1 (Class 5) work area ...

Biological safety cabinet class III
Specially designed for the handling of microbiological agents, when working with indige...
Hygienic floor scale with lifting device
The food and pharmaceutical industries require maintaining high levels of sanitati...

Flexible contained powder charging solution
Filling of transportation bags and discharging them into mix tanks or reactor ...

Flexible contained charging for split butterfly valves
Poly bottles with split butterfly valves are often used to transfe...

Dosing machine for nonfree-flowing powders
Fill poorly flowing powders, dry syrups and similar substances into various gla...

Restricted-access barrier systems
Create a physical barrier between the operator and the product. More flexible than an iso...

Flexible contained handling of powder transfer bags
Filling of transportation bags and discharging them into mix tanks or...

Crimping system
Flexible disposable containment systems in the form of specially designed plastic bags are an excellent choice...

Flexible contained drum transfer system
Transferring powders from drums to process equipment such as reactors or mills can ...

Flexible contained powder discharge into FIBC’s
Flexible intermediate bulk containers known as FIBC’s are commonly used in...

Flexible contained powder discharge into drums
Powdered products are handled every day in the pharmaceutical and bioproces...

Checkweigher for stand-up pouches
When working with several lines of stand-up pouches, you can benefit from controlling the...








