Making Hpmc Capsules
Find innovative production technology for making hpmc capsules and connect directly with world-leading specialists
The nonproprietary name for HPMC – hydroxypropyl methylcellulose – is such a mouthful that it has two abbreviations. Hypromellose is the other one. The non-ionized material offers a safe alternative to gelatin capsules, especially as most common API and excipients are suitable HPMC capsule ingredients.
Select your hpmc capsules process
Tell us about your production challenge
HPMC manufacturing equipment must operate at higher temperatures
HPMC is one of many types of pharmaceutical capsules. As a result, it will require slightly different production processing. HPMC capsules is produced using a dipping-pin method, similar to the technique used to produce gelatin capsules. But the Hypromellose solution needs to be kept at higher temperatures (around 70°C), and shells take longer to form on the manufacturing pins.
Moreover, the structure of HPMC capsules is significantly more fragile than that of gelatin. Processing with drying and filling equipment must therefore operate at a gentler pace.
Hygroscopic materials are compatible HPMC capsule ingredients
One limitation of traditional animal-based gelatin is that absorbent and adsorbent materials gradually weaken the shell walls.
On the other hand, HPMC capsules offer superior stability at a broader range of heat and moisture variances. This makes them better suited for hygroscopic formulations such as zinc chloride or sodium hydroxide.
HPMC opens opportunities for the production of vegan enteric-coated tablets
The generic capsule owes its success as a pharmacological product to the efficacious delivery of drugs. HPMC capsules are not only as easy to swallow as hard-gel alternatives, but they carry an identical dissolution rate in-vivo.
In addition, the matt properties of Hypromellose make it a viable option for delayed and extended-release medical formulations. HPMC, thus, shows promise for the manufacturing of plant-based enteric-coated tablets.
Processing steps involved in HPMC capsules making
Which hpmc capsules technology do you need?

High throughput capsule filling system
Achieve precise, high-speed capsule filling with versatility, ensuring product integ...
Empty capsule sorting system
Streamline your capsule production by efficiently removing empty and defective capsules, ensuri...

Capsule polisher for pharmaceutical production
Enhance capsule quality and safety by effectively polishing, dedusting, and...

Capsule polisher for pharmaceutical capsules
Ensure your capsules are free from dust and perfectly polished with a solutio...

Capsule selection and feeding unit
Enhance your capsule filling process with precise selection and feeding, ensuring only t...

Precision weight-checking for pharmaceutical capsules
Ensure exact capsule and tablet dosage with high-speed precision we...
Blister packaging system for tablets and capsules
Designed for seamless 24/7 production, this blister packaging machine e...

High-speed blister packaging with integrated cartoner
Optimize your blister packaging process with a solution that seamle...

Capsule filler for multi-product dosing
Achieve precision and flexibility in capsule filling with a system designed for hig...

High-speed capsule weighing system for quality assurance
Ensure precise capsule weight monitoring and sorting with a syst...

Capsule filling system for powder, pellets, and tablets
Optimize capsule production with rapid dosing changes, minimizing...

Versatile capsule filler for pharmaceuticals
Effortlessly switch between powder, pellet, and liquid dosing with this adapt...

Filled capsule conveyor system for pharmaceutical production
Ensure gentle and efficient conveying of filled capsules in...

Laboratory film coating system for development and clinical batches
Achieve precision in tablet coating with a versatil...

High-speed strip packaging for tablets and capsules
Optimize your high-speed pouching operations with precise four-side s...

Capsule filling solution for small to medium batch sizes
Achieve precise and efficient capsule filling up to 3,400 units ...

Flat-forming blister packager for small production batches
Ideal for pharmaceutical and nutraceutical firms seeking effi...

Automatic capsule checkweigher for statistical weight control
Ensure precise weight control of capsules with automatic s...

Capsule de-dusting and polishing system
Ensure precision in capsule production with continuous de-dusting and polishing, en...

Empty capsule sorting system for pharmaceutical manufacturing
Expedite your capsule production with a high-speed system ...

Capsule sorter elevator for encapsulation processes
Ensure uninterrupted encapsulation by efficiently removing defective ...

Flat forming and sealing blister packaging solution
Enhance your blister packaging process with a solution that ensures p...

Capsule filling for pharmaceuticals and nutraceuticals
Enhance your capsule production capabilities with modular filling ...

Capsule printer for pharmaceutical applications
Experience precise dual-color printing with high-speed efficiency for caps...
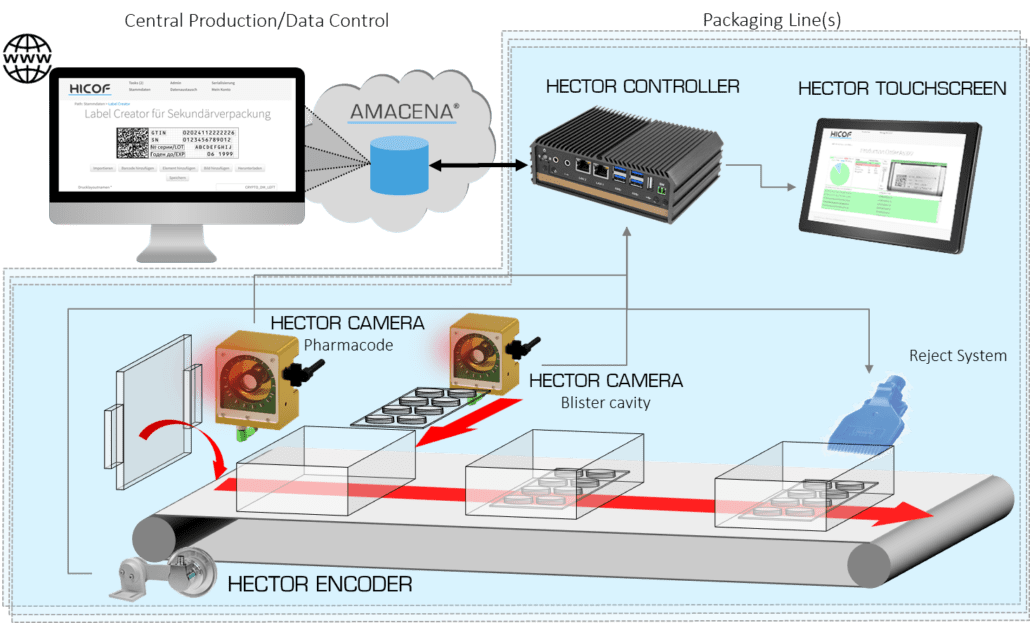
Serialization and aggregation inspection system
Including serialization and aggregation inspection systems to packaging li...

Vision inspection system for tablets and capsules
Pharmaceuticals and healthcare product manufacturers dealing with oral ...

Serialized labels inspection and printing station
Labels are a key component in pharmaceutical packaging lines as they in...

Easy to validate capsule filler
Staying competitive in today’s highly dynamic and highly regulated pharmaceutical prod...

High speed bottle filler with inspection
Medicines in tablet, capsule, or soft gel forms undergo various processes before r...

Effective Visual Inspection System for Capsules
The attribute of capsule packing is a critical parameter that controls the...

Lifter and De-duster for Tablets
The use of several different tools for lifting, de-dusting, and polishing in the pharmaceut...

High Speed Visual Inspection System for Tablets
In order to ensure quality and standard specifications of tablets, capsule...

Tablet counting machine
Production of pharmaceutical solid dosage forms usually require complete accuracy, flexibility, and c...

Pharmaceutical bottle unscrambler
Pharmaceutical packaging industry requires the ability to quickly manage the process yet ...

Dessicant inserting machine
Desiccant canisters play an important role in the pharmaceutical and nutraceutical industries. T...

Metal detector for bottles
For metal contamination detection in finished products that are bottled, traditional solutions re...

Automatic capsule loader
Capsules are a popular method of dosage delivery for a wide range of compounds. For anything other t...

Electronic capsule counter
For lab work or small batch production, hand counting of solid doses into dispensing containers i...

Semi-automatic case packer
Pharmaceutical products require complete traceability throughout the production cycle. For smalle...

Automatic laboratory capsule filler
Innovators in the highly regulated and highly dynamic pharmaceutical market place need ...

Automatic capsule filler
Capsule filling should be a worry-step in pharmaceutical and nutraceutical manufacturing. The right...

Industrial capsule checkweigher
Pharmaceutical manufacturing demands the highest quality standards and completely consistent...

Capsule checkweigher
The production of pharmaceutical capsules requires that individual doses are checked for accuracy. In a ...

Stand-alone aggregation station
The aggregation of pharmaceutical products for tracking purposes is already compulsory in ma...

Serialization coding and labeling equipment
In the pharmaceutical industry, product serialization is the cornerstone of al...

High-yield capsule filler
Pharmaceutical manufacturers require maximum yield from their encapsulation process for efficient ...

High capacity capsule filler
In the highest volume pharmaceutical production environments, capsule filling needs to be extre...

Scale up capsule filler
For higher production volumes of pharmaceutical capsules, speed, accuracy and reliability of producti...

Entry level capsule filler
For the production of pharmaceutical doses in capsule form complete accuracy of fill is paramount...

R&D semi-automatic capsule filler
Semi-automatic filling machine suitable to open, fill and close empty hard gelatin capsul...

R&D hard gelatin capsule sealing machine
When encapsulating products at temperatures up to + 80°C, these tend to solidify a...

R&D electronic counter for capsules and tablets
This machine has been designed to offer an ever-precise and reliable count...

R&D automatic capsule filler
Automatic capsule filling machine for powders, pellets, tablets, mini-tablets, capsule in capsu...

R&D capsule orientater
A semi-automatic orienter for capsules with an output speed up to 6000 capsules.

Checkweigher for capsules
The new interactive indicator unit allows for even easier use. High-rigidity weighcells have been ...

Checkweigher
A check weigher that is equipped with high speed/high accuracy force balance load cell. It meets stringent accura...

High-sensitivity metal detection system
Highly efficient metal detector with enhanced quality control and reporting capabil...

Entry-level X-ray inspection system
Without compromising on performance, if you need an entry level x-ray inspection system...