Making Nitrogen Fertilizer
Find innovative production technology for making nitrogen fertilizer and connect directly with world-leading specialists
Growing populations and intensive farming in the last centuries reduced soil nitrate levels. Aware of the risks to food security, chemists Fritz Haber and Carl Bosch developed a way to industrialize synthetic nitrogen fertilizer production. They created the Haber-Bosch process for ammonia synthesis which is still used today. Manufacturers produce over a hundred million metric tons of nitrogenous fertilizers every year.
Select your nitrogen fertilizer process
Tell us about your production challenge
The principal ingredient in nitrogen fertilizer production is Ammonia
Nitrogen fertilizers (N-fertilizers) are nitrous compounds used to improve crops’ growth, texture, and quality. Nitrogen fertilizer production starts by combining the nitrogen from the air with the hydrogen in a natural gas, usually methane. The resulting mix – Ammonia – is formed in a reactor at high temperature and pressure. Ammonia is sometimes applied directly as fertilizer, but its primary purpose is for manufacturing fertilizer. You can reuse a percentage of it to produce electricity and heat.
After this first step, ammonia is used to make nitric acid, urea, and ammonium nitrate for feedstock. Mix it with other ingredients to produce other fertilizers such as NPK’s (nitrogen, phosphorus, potassium) and UAN (urea ammonium nitrate). The industry also uses sewage, manure, and agriculture by-products to make N-fertilizers.

Develop the nitrogenous process into dry or liquid fertilizers
N-fertilizers are typically produced in either dry or liquid forms. Dry fertilizers are made with pellet mills or crumblers, also known as granular fertilizers. They are slow-releasers and may be made from an organic or natural source like cottonseed meal powdered fertilizer. Dry fertilizer obtained from chemical reactions, such as urea, cannot be used in organic farming.
On the other hand, liquid fertilizers provide nutrients in a faster way. Most of them are diluted in water and are spread more evenly. They are transported and stored in special tanks to avoid leakage and environmental damage.
Regulations control nitrates concentration to prevent environmental damage
N-fertilizers have many benefits and a considerable risk of causing substantial ecological damage. Nitrogen is about 300 times more polluting than carbon dioxide. If applied in excess, it causes land degradation, air pollution, and contamination of groundwater sources.
Many jurisdictions, including Europe, the USA, China, and Australia, enacted laws to regulate N-fertilizers in farming and prevent nitrates concentration and environmental damage. A significant challenge in the sector is to produce greener nitrogen fertilizers and apply them more efficiently and sustainably.
Processing steps involved in nitrogen fertilizer making
Which nitrogen fertilizer technology do you need?

Perforated plate sieves for particle size determination
Optimize your particle size separation with high-stability stainl...

High shear emulsifier for mayonnaise production
Streamline your production of high-quality emulsions with this high-capaci...
Cheese blending and heating line for processed cheese
Optimize your processed cheese production with a solution that seam...

Industrial cooker for sauces and stews
When producing diverse culinary delights such as sauces and stews, achieving even he...

Industrial cutting system for fine emulsions
Optimize your production line with precision cutting and emulsifying, ensurin...

Vacuum deaeration system for mustard and liquid detergents
Ensure optimal product quality by effectively removing air fr...
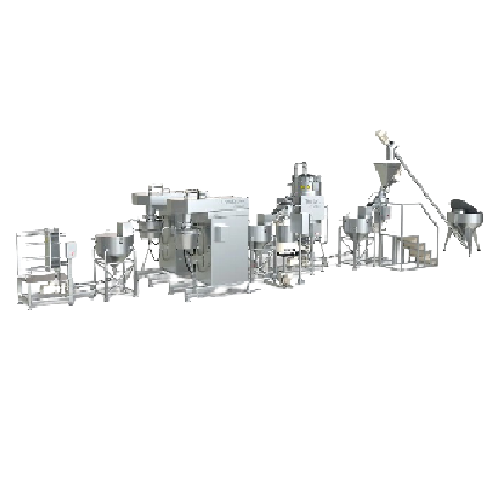
Continuous tahina production line
Streamline your tahina production with precise grinding and controlled cooling, ensuring ...

Discharge systems for difficult bulk materials
Achieve seamless bulk material discharge with our advanced systems that eff...

Automatic bulk material filling system
Ensure precise, contamination-free filling of various bulk materials with an advance...

High-viscosity continuous industrial kneader for small volumes
Achieve consistent high-viscosity material processing wit...

Packed and tray columns for distillation, absorption, and extraction
Enhance your production line with columns designed...

Protein and nitrogen analysis system for laboratories
Streamline your nitrogen and protein analysis with precision titrat...

Ethylene recovery unit for ethylene oxide and glycol production
Recover valuable ethylene monomer efficiently from cycle...

Food product sifter
Ensure precise separation with vibratory sifters designed for continuous operation, delivering gentle han...

Atex-certified flanged polygonal dust collectors
Ensure dust control and compliance in explosive environments with our com...

Industrial dust collection system for air filtration
Optimize air quality in your production line with this compact dust ...

Fit-frame butterfly valves for dry bulk solids
Achieve precise control and minimize contamination in your dry bulk materia...

Low profile slide valve for controlling powder flow
Optimize your powder and granule flow management with precision-engin...

Diverter valves for pneumatic conveying lines
Experience precise flow control in pneumatic conveying with diverter valves ...

Drum-type diverter valves for pneumatic conveying
Optimize your pneumatic conveying system by effortlessly controlling th...

Blow-through rotary valves for pneumatic conveying
Optimize your pneumatic conveying systems with high-efficiency blow-th...

Rotary ball vibrators for bulk solids discharging
Ideal for enhancing material flow, this equipment efficiently handles f...

High flow rate Fibc discharger
Optimize your bulk material handling with a system designed for efficient and dust-free FIBC ...

Rotary turbine vibrators for bulk solids discharging
Enhance the efficiency of material handling with high-speed, low-noi...

Continuous impact vibrator for aggregate reclaiming
Tackle material flow challenges head-on by preventing common issues l...

External electric motovibrators for industrial applications
Optimize material movement and improve discharge efficiency ...

External electric motovibrators for hazardous materials
Ensure safety and efficiency in hazardous environments with relia...

Silo overfilling safety system
Ensure safe silo filling with our system that prevents overfilling and excess pressurization,...

Cushioned pneumatic linear vibrators for bulk solids
Combat material bridging and rat-holing with silent vibratory techno...

Anti-wear elbows for pneumatic conveying systems
Reduce wear and extend the lifespan of your pneumatic conveying systems w...
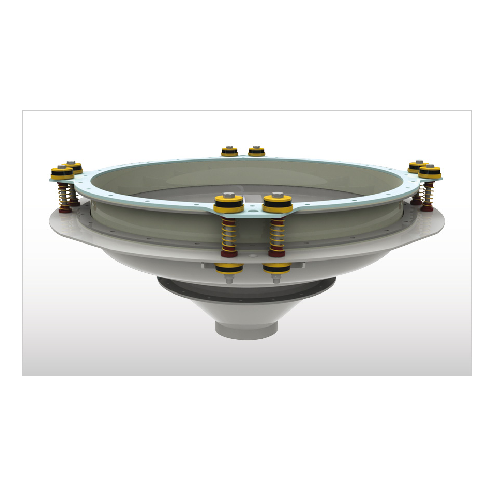
Industrial bin activator for smooth material flow
Enhance your material handling process with a solution that ensures con...

Bin activator for silo and hopper discharge
Ensure optimal material flow and prevent blockages in your storage systems wit...

Laboratory bead mill for sample dispersion
Ensure precise particle size reduction with versatility and ease, enabling effi...

Vibrating screen for sticky and moist materials
Efficiently tackle the challenges of processing wet and sticky materials w...

Dynamic and static weighbridge for rail cars
Ensure precise legal-for-trade weighing and data recording of rail cars throu...
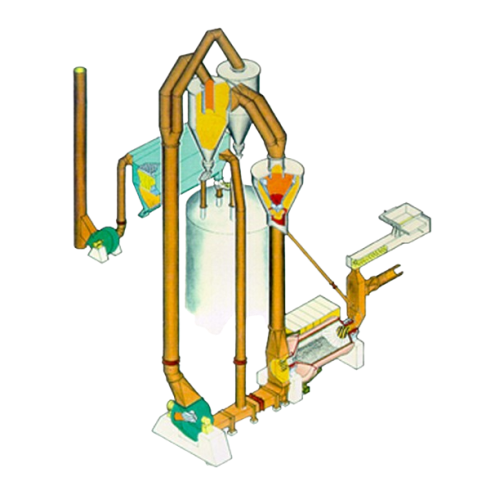
Industrial grinding solution for hard and abrasive materials
Efficiently grind and mill a spectrum of materials from coa...

Loss-in-weight feeder for poor flowing bulk solids
Efficiently handle poorly flowing bulk solids with unparalleled precis...

Bead mill for crop protection
To optimize qualities like particle size, distribution, solubility, and miscibility of active ...
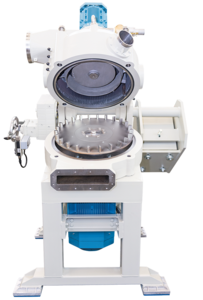
Classifier mill for recovered carbon black
Recovered Carbon Black (rCB) can be recycled back to the tire production chain ...

Classifier mill for powder coating
High-quality powder coatings are often required to manufacture equipment, appliances, an...

Automatic palletizer machine for bags and boxes
The effective placement and positioning of bags and boxes can be a challen...

Packaging machine for sealed plastic bags from 250 g to 10 kg
Sealing small quantities of solids or liquids into plastic...

Big bag discharge system
Dust emissions, product loss and product contamination are the main risks when discharging products ...

Light duty rotary dust valve
In some light industrial applications there is limited pressure differential for valve operation.

Heavy duty rotary valve
Handling powdered and granulated materials in pneumatic conveying systems requires consistent, safe v...

Special duty horizontal metering valve
Occasionally, there is no suitable standard valve available for a particular conveyi...

Dual channel plug diverter valve
Short switching times are often required when diverting or combining powder or granular sol...

In-process weighing system for mills
When you need a throughput weigher for modern flour/grain milling applications, the we...

Robot palletizer
Palletize up to 1,800 large industrial bags per hour at a multi-pick-up configuration.

Small scale pelleting press
Many industries require reliable pelletizing equipment with smaller scale production capacities....