Contamination-free docking system for bulk solids
Achieve contamination-free transfer of bulk powders and granules while ensuring precise dosing and weighing. Ideal for maintaining product integrity and operational efficiency, this solution seamlessly integrates into existing production lines, reducing dust emissions and minimizing cross-contamination risks.
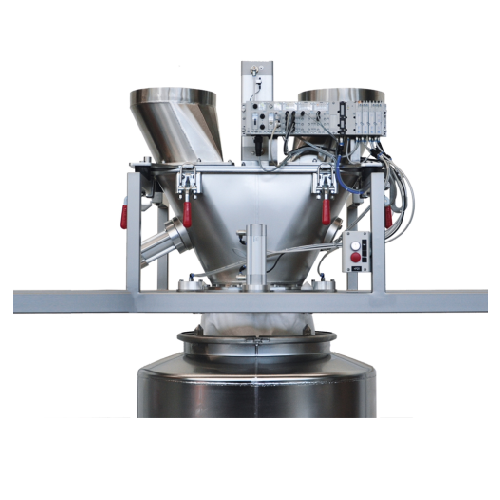
Ensures Contamination-Free Docking and Precise Weighing
The AZO CleanDock® from AZO GmbH & Co. KG is a docking system designed for contamination-free material transfer in industries like pharmaceuticals, food, and chemicals. This system automates the docking and undocking of containers, ensuring dust-tight connections that protect both product and operator. By integrating scale decoupling, it delivers precise dosing and weighing, crucial for applications such as producing pharmaceutical tablets and nutritional supplements. The system’s low dust emissions eliminate the need for ATEX zones, streamlining compliance. Engineered for ease of cleaning, the CleanDock® features quick release fasteners and optional mobility, allowing for in-situ cleaning without disrupting production. It operates on a batch processing basis, with high accuracy and seamless integration into existing lines, tailored for those handling challenging powder and granule materials.
Benefits
- Enhances product integrity and safety with contamination-free docking and undocking.
- Improves dosing accuracy by decoupling scales from mechanical forces, ensuring precise weight control.
- Eliminates costly ATEX compliance at transfer points due to negligible dust emissions.
- Minimizes production interruptions with quick, on-site cleaning and maintenance.
- Increases operational flexibility with optional mobility for diverse production line setups.
- Applications
- Vitamins, Supplements, Metal powders, Fine chemicals, Detergents, Pet food, Nutraceuticals, Sweets, Enzymes, Spices, Minerals, Baby food, Pharmaceutical powders
- End products
- Mineral supplements, Nutritional bars, Laundry detergent powder, Specialty chemicals, Pharmaceutical tablets, Enzyme blends, Infant formula, Vitamin c tablets, Metal powder alloys, Candy-coated chocolates, Kibble, Protein supplements, Curry powder
- Steps before
- Loading, Container Preparation, Positioning Hopper
- Steps after
- Cleaning, Product Transfer, Repositioning Hopper
- Input ingredients
- mobile containers, hoppers, drums, big bags, bulk materials
- Output ingredients
- exactly dosed bulk materials, weighed bulk materials
- Market info
- AZO is renowned for designing and manufacturing automated bulk material handling systems, specializing in mixer feeding, ingredient automation, and process control, known for their reliability and tailored solutions in the food, pharmaceuticals, and chemical industries.
- Dosing Accuracy
- High degree due to scale decoupling
- Dust Emissions
- Low
- Docking Type
- Automatic
- Undocking Type
- Automatic
- Container Compatibility
- Hoppers, drums, big bags
- Cleaning Method
- Quick release fasteners, in situ
- Scales Decoupling
- Integrated
- Switching Flow
- Coarse to fine flow by motor speed reduction
- Seal Type
- Dust-tight
- Contamination control
- Sealed system
- Automation level
- Automatic docking/undocking
- Cleaning method
- Quick release for in-situ cleaning
- Batch vs. continuous operation
- Batch
- Dosing accuracy
- High degree with scale decoupling
- Dust emission
- Low emissions
- Cleanability
- Easy to clean design
- Docking method
- Dust-tight connection
- ATEX certification
- Not necessary to define ATEX zones due to low dust emissions
- CE marking
- Compliant
- GMP Compliance
- Likely compliant due to contamination prevention features
- 3A Sanitary Standards
- Potentially applicable based on clean design
- FDA compliance
- Applicable based on prevention of contamination and cleanability
- EHEDG certification
- Potentially applicable given the easy-to-clean design
- Discharge method
- Automatic docking and undocking
- Compact footprint
- Design ensures minimized space usage
- Container compatibility
- Hoppers, drums, big bags
- Sealed System
- Dust-tight connection
- Mobility
- Option for mobile design
- Control panel type
- Optional mobile design
- Mobility
- Mobile design available
- Integration possibilities
- Dosing device integration
- Cleaning method
- In situ
- Quick release fasteners
- Docking type
- Automatic docking and undocking
