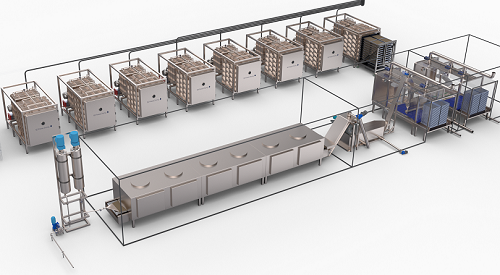
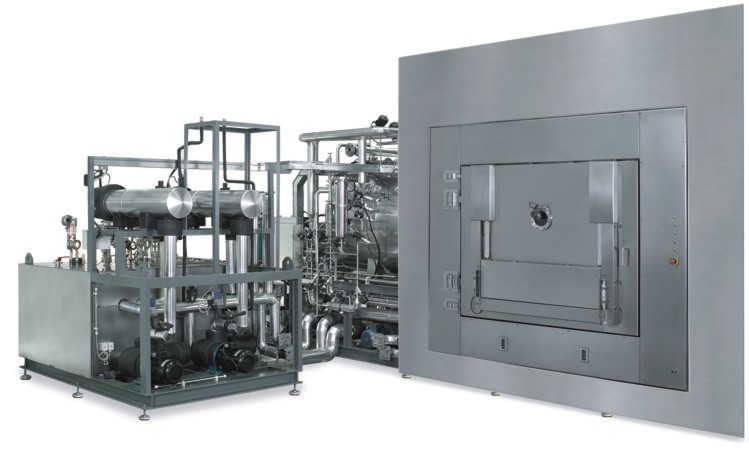
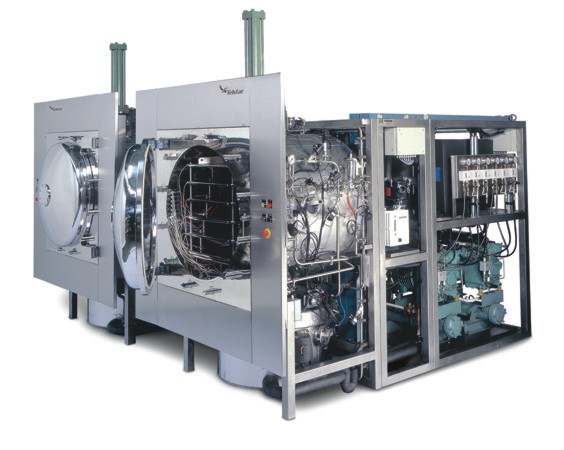
Pharmaceutical freeze drying solutions
Lyophilisation (freeze drying), is used for dehydrating high-value products that are too sensitive for conventional drying processes. Perishable wet materials, like food and pharmaceuticals, are preserved so that they are more stable and the risk of spoiling by micro-organisms is reduced. Also, the final product can be stored and handled much more easily.
Freeze Drying Applications
Due to the low temperatures used in freeze drying, damage is avoided to heat sensitive products such as enzymes, flavours, proteins and vitamins. In pharmaceutical applications, freeze drying improves the stability and long term storage life of labile drugs, especially protein drugs. In the pharmaceutical industry, freeze drying is used for preserving and storing high value products such as vaccines, cytostatics, antibiotics, biologicals, hormones, active ingredients and reactives. It is also used for collagens, APIs and electrolytes.
The material to be dried must first be frozen to well below its triple point since any remaining liquid will destroy the structure once drying begins. The pressure around the material is then lowered, to sublimate the ice from the solid phase, directly into the gas phase, without becoming liquid. As the ice leaves the material the structure is left behind, intact.
- Pre-treatment – where components are added to the material to be freeze dried. This is done mainly to increase the yield, quality and appearance of the final product, but also to increase the temperature of drying and reduce cycle time, if possible.
- Freezing – the size of the ice crystals has a significant effect on the quality of the final freeze dried product. Large crystals dry faster, but in forming larger structures, biological material can be destroyed.
- Primary drying – during this step, the pressure inside the drying chamber is lowered and a very small amount of heat is applied. This step can take days in an industrial freeze drying cycle, however, if too much heat is applied the structure could be altered or destroyed.
- Secondary drying – is the final attempt to remove remaining water molecules, since all the ice should have been removed in the primary phase. Pressure is usually further reduced and the temperature increased.
As seen in the above steps, freeze drying is a time and energy intensive process that can take days or even weeks to finish if the freeze drying cycle is not optimized. A non-optimum process may compromise drug stability, take longer, and cost much more than necessary. For this reason, our range of drying equipment includes pilot and laboratory dryers. These enable the user to maximize the yield of the product at optimum production cost on a small laboratory or pilot plant scale, enabling a smooth transition to production plant. For more information on our full range of specialist industrial freeze drying solutions, get in touch our industry experts and and tell us about your production process and application. We look forward to hear from you!