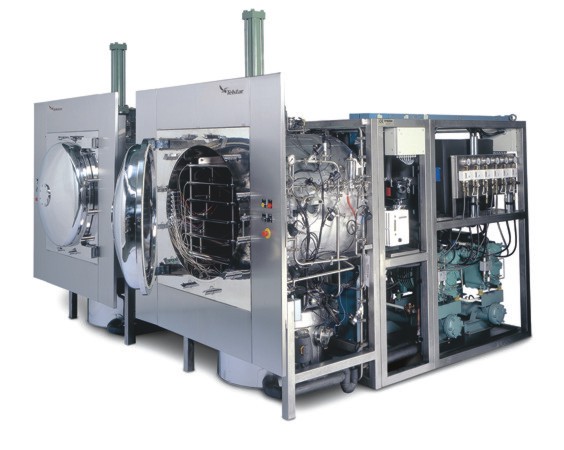
GMP freeze dryer
Designed to perform clinical trials, pilot, and small scale or large scale industrial drying processes, in accordance with the strictest GMP and Regulatory standards and in line with the latest freeze drying technology trends.
The right balance between specification, quality, cost and lead-time
The Lyonomic Series from Telstar Life-Sciences are state of the art freeze-drying systems, designed and engineered modularly to provide a comprehensive range of standard models and in-factory options.
Telstar freeze drying shelf inner channel design ensures high product homogeneity during the freezing, primary and secondary drying stages. The SCADA supervisory, control and data acquisition system designed by Telstar, allows the user to readily interpret the machine’s operation and obtain information on the process required for decision making and batch reporting, all in compliance with FDA 21 CFR part 11 electronic record guidelines.
Benefits
- Covers freeze drying areas from 4 m2 to 50 m2
- Easy maintenance access to all elements and optimizing the total space necessary
- Ensures high product homogeneity during the freezing, primary and secondary drying stages
- Allows the user to readily interpret the machine's operation and obtain information on the process required for decision making
- In compliance with FDA 21 CFR part 11 electronic record guidelines



