Freeze dryer for small-scale production
Optimize your production line with reliable lyophilization that ensures precise moisture removal for pharmaceuticals, enhancing stability and shelf life in vials, ampoules, and syringes.
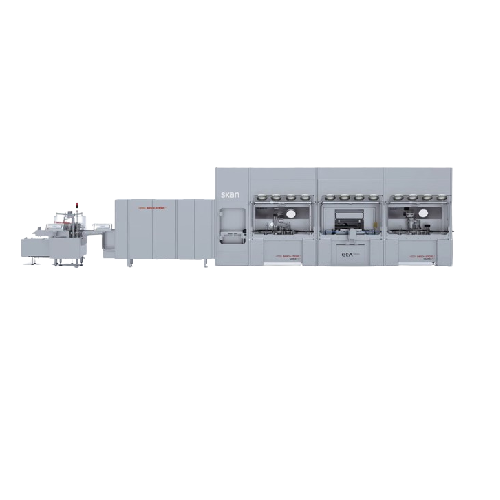
Performs Precise Lyophilization for Pharmaceuticals
The LYOVAC® FCM Series from GEA provides pharmaceutical manufacturers with freeze dryers designed for small-scale and pilot production of complex drug formulations. These units excel in precise lyophilization, handling products like vaccines, monoclonal antibodies, and diagnostic reagents with efficiency and reliability. Equipped with an integrated VarioSys® Isolator system, they ensure safe handling of potent substances. The series includes models with shelf areas of 1.5 m², 4.5 m², and 9.9 m², processing up to 42,406 vials per batch. Each unit is ATEX-compliant and operates efficiently in Grade C environments. Automation features such as the ALUS® system facilitate seamless loading and unloading, while LYOSPARK® controlled nucleation ensures consistent product quality. With options for partial loading and compatibility with a range of container types, these freeze dryers support flexible production needs. Compliance with stringent GMP standards ensures readiness for pharmaceutical and biotech applications.
Benefits
- Enables versatile production with flexible batch sizes and partial loading.
- Enhances operator safety and product integrity through integrated isolator systems.
- Reduces contamination risk with automated loading and unloading.
- Maintains product consistency using controlled nucleation technology.
- Complies with GMP standards for pharmaceutical applications.
- Applications
- Liquid formulations, Syringes, Lyophilized products, Vials, Biopharmaceuticals, Ampoules, Cartridges, Powder formulations, Pharmaceuticals
- End products
- Biologic injectables, Insulin vials, Injectable nutrients, Vaccines, Lyophilized enzymes, Monoclonal antibodies, Antiviral ampoules, Mrna therapeutics, Gene therapy solutions, Diagnostic reagents, Hormone syringes, Oncology drugs, Blood product vials, Sterile water cartridges, Parenteral nutrition solutions, Hydrocortisone freeze-dried powders, Freeze-dried plasma, Antibiotic powders
- Steps before
- Filling, Capping
- Steps after
- Packing, Storage, Distribution
- Input ingredients
- vials, liquid, powder, volatile products, toxic substances, potent substances
- Output ingredients
- lyophilized products, freeze-dried products, ampoules, syringes, cartridges
- Market info
- GEA is known for its expertise in engineering innovative and sustainable equipment and solutions, focusing on sectors such as food, beverages, pharmaceuticals, and energy, with a reputation for quality, efficiency, and advanced technological applications in industrial processing.
- Compressor capacity
- 25 kg, 75 kg, 150 kg
- Shelf area
- 1.5 m², 4.5 m², 9.9 m²
- Vial processing capacity
- 6500 (2 mL) for FCM 25-I, 20, 000+ for FCM 75-I, 42, 000+ for FCM 150-I
- Automation
- Automatic loading and unloading (ALUS®)
- Compliance level
- QA standards (GMP)
- Operating environment
- Grade C environments
- ATEX-compliance
- Yes
- Control feature
- LYOSPARK® controlled nucleation
- Detection feature
- LYOPLUS® silicone oil detection
- End-point detection
- Alternative available
- System integration
- Integrated VarioSys® Isolator
- Batch flexibility
- Partial loading possible
- Turnaround time
- Fast through defrost/CIP/SIP
- Product compatibility
- Toxic, potent, volatile products
- Product forms
- Liquid, powder, lyophilized
- Batch operation
- Yes
- Automation level
- PLC / SCADA
- CIP/SIP
- Defrost/CIP/SIP
- Cleaning method
- CIP
- Compact footprint
- Yes
- Changeover time
- Fast turnaround
- Energy efficiency
- ATEX compliant
- Process flexibility
- Nests and tubs
- Modular integration
- VarioSys® Isolator
- Production scale
- Small scale
- LyoSpark® controlled nucleation
- Yes
- Corrosive resistance (e.g. acids)
- Yes
- Cleanability
- Easy to clean
- Biological compatibility
- Pharmaceutical grade
- Density/particle size
- Appropriate for Freezing applications
- Abrasion resistance
- Yes
- Application Range
- Vials, Ampoules, Syringes, Cartridges
- Product Forms
- Liquid, Powder, Lyophilized
- GMP Compliance
- Yes
- ATEX certification
- Yes
- Machine footprint
- Compact
- Shelf area
- 1.5 m² / 4.5 m² / 9.9 m²
- Ice condenser capacity
- 25 kg / 75 kg / 150 kg
- Batch capacity
- 6500 vials / 20, 000 vials / 42, 000 vials (2 mL)
- Design for GMP compliance
- Yes
- Integration with VarioSys
- Yes
- Partial loading capability
- Yes
- Automatic Load,Unload System (ALUS®)
- Available
- Belly condenser design
- Yes
- Grade C environment suitability
- Yes
- Control panel type
- Touchscreen / PLC
- Integration possibilities
- VarioSys® Isolator
- Footprint design
- Compact with belly condenser








