Continuous direct compression system for tablet production
Achieve seamless continuous direct compression by integrating dosing, blending, conveying, and compression in one compact system, optimizing space and efficiency in your tablet production line.
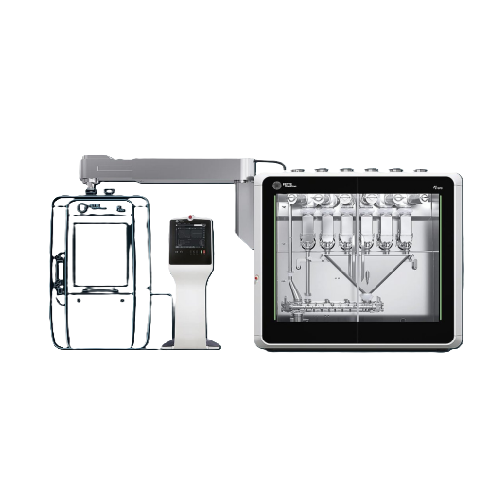
Performs Continuous Dosing, Blending, and Tablet Compression
The FE CPS from Fette Compacting is an innovative continuous processing system designed for pharmaceutical applications. What sets the FE CPS apart is its seamless integration of dosing, blending, conveying, and compression within a compact footprint. This system actively supports the production of pharmaceutical tablets and powders, such as Ibuprofen and Vitamin C tablets, handling throughput capacities ranging from 10 to 200 kg/h depending on formulation. Operating as a continuous process, it features embedded Process Analytical Technology (ePAT) for real-time process monitoring and quality control.
This ensures precise adjustments can be made swiftly to maintain product consistency and quality. With flexible setup options and modular design, the FE CPS can be installed in various configurations to fit existing production lines. Constructed with materials that prioritize operator safety and ease of maintenance, it also includes dust-tight units with negative pressure systems for safe handling of pharmaceutical powders. Furthermore, its user-friendly Human Machine Interface (HMI) simplifies process management, offering centrally controlled parameters for all operations. The FE CPS system complies with GMP standards, making it suitable for pharmaceutical manufacturing environments aiming to enhance efficiency and reduce production complexity.
Benefits
- Enhances production efficiency with continuous high-speed processing.
- Ensures consistent product quality with real-time process monitoring.
- Simplifies cleaning and changeovers with modular, easily accessible components.
- Increases safety and maintenance ease with dust-tight, negative-pressure design.
- Integrates seamlessly into existing facilities with flexible setup configurations.
- Applications
- Vitamins, Supplements, Pharmaceutical tablets, Nutraceuticals, Pharmaceutical powder
- End products
- Ibuprofen tablets, Herbal supplement capsules, Vitamin c tablets, Calcium supplement tablets, Protein powder
- Steps before
- Dosing, Blending, Conveying
- Steps after
- Tablet Pressing
- Input ingredients
- formulations, raw materials
- Output ingredients
- tablets, finished product
- Market info
- Fette Compacting is renowned for its advanced tablet press technology, offering high-performance machines and process equipment for the pharmaceutical and nutraceutical industries, emphasizing innovation, quality, and efficiency in tablet production.
- Automation
- Fully integrated
- Throughput Capacity
- 5–200 kg/h
- Dimensions
- 5 x 6.5 meters footprint
- Compression Stations
- Three compression stations
- Control System
- Single central control with EPT technology
- Operating Height
- 2, 300 mm
- Operating Width
- 2, 650 mm
- Operating Depth
- 925 mm
- Weight
- Approx. 5, 000 - 5, 500 kg
- Cleaning Method
- Fast and easy product changeover
- Batch vs. continuous operation
- Inline Continuous
- Automation level
- Integrated HMI / ePAT system
- Changeover time
- Extremely fast
- CIP/SIP
- Easy cleaning
- Reduced system components
- Cleaning method
- Manual
- Easy to clean design
- Process integration
- Dosing-blending-conveying
- Footprint
- 5 x 6.5 meters
- Abrasion resistance
- High
- Biological compatibility
- Pharmaceutical-grade
- Cleanability
- Easy CIP/SIP
- Corrosive resistance (e.g. acids)
- Stainless steel construction
- Density/particle size
- Varies based on formulation
- Process type
- Continuous Direct Compression
- Machine footprint
- 5 x 6.5 meters
- Dimensions
- Height
- Weight
- approx. 5, 000 - 5, 500 kg
- Setup options
- Vertical or horizontal installation
- Product changeover capability
- Fast and easy
- Discharge method
- Continuous dosing-blending-conveying
- Control panel type
- Human Machine Interface (HMI)
- Flexible installation
- Vertical or Horizontal Setup
- Integration possibilities
- Continuous dosing-blending-conveying system with tablet press
- Process area separation
- Separate process and technical areas
- Cleaning accessibility
- Components easily removable and cleanable