Sterile lyophilisation and filling line
Ensure aseptic conditions while freeze-drying biopharmaceuticals and high-potent drugs with seamless integration into your filling line, maximizing product sterility and handling efficiency.
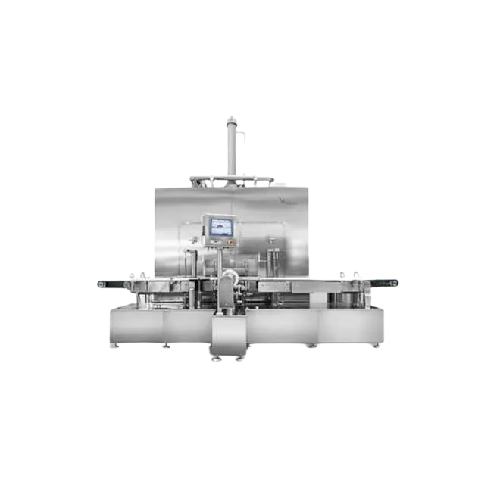
Processes and Lyophilizes Aseptic Vial Fillings
The Sterile Lyophilised Vial Filling Line from Telstar offers cutting-edge solutions for pharmaceutical and biotech companies needing precision in aseptic processing. This line integrates seamless vial filling with advanced lyophilization, ideal for high-potent drugs like monoclonal antibodies and mRNA vaccines. Featuring an automatic row-by-row single-side push-in/pull-out system, it ensures efficient loading and unloading. The system incorporates SCADA for comprehensive process control and real-time monitoring, optimizing production efficiency. Designed with a mushroom-type chamber-condenser isolation valve, it excels in solvent handling and vacuum processing, minimizing contamination risks. The equipment meets stringent GMP standards, ensuring compliance for pharmaceutical applications. With integrated CIP and SIP systems, it offers enhanced cleaning and sterilization efficiency, reducing downtime and ensuring product safety. Customization options cater to specific production needs, supported by expert engineering support from Telstar.
Benefits
- Ensures product safety with GMP-compliant aseptic processing.
- Enhances operational efficiency with SCADA-integrated real-time monitoring.
- Minimizes contamination risks using advanced solvent handling and isolation systems.
- Reduces cleaning time and contamination with integrated CIP and SIP systems.
- Customizable configurations provide flexibility for diverse pharmaceutical applications.
- Applications
- High potent drugs, Vaccines, Freeze-dried products, Vials, Aseptic products, Biopharmaceuticals, Pharmaceuticals
- End products
- Cytotoxic drugs, Lyophilized diagnostic kits, Lyophilized vial fillings, Antibiotics, Monoclonal antibodies, Injectable insulin, Mrna vaccines, Parenteral nutrition solutions, Freeze-dried plasma, Sterile injectable solutions
- Steps before
- Vial preparation, Solution filling, Solvent handling
- Steps after
- Sterilization, Aseptic sealing, Packing
- Input ingredients
- vials, high potent drugs, solute, solvent
- Output ingredients
- sterile lyophilised products, freeze dried vials, lyophilised drugs
- Market info
- Telstar is known for specializing in the design, engineering, and manufacturing of advanced vacuum and aerospace solutions, gaining a strong reputation for their innovative and customized equipment in pharmaceutical, medical, and scientific research sectors.
- Freeze dryer size
- 1 X 20 m²
- Ice condenser capacity
- 400 kg
- Solvent handling configuration
- Yes
- Chamber-condenser isolation valve
- Mushroom type
- Cleaning in Place (CIP)
- Yes
- Sterilization in Place (SIP)
- Yes
- Dry vacuum pumps
- Yes
- Loading/unloading system
- Automatic row by row single side push-in/pull-out
- Control system
- SCADA control package
- Working Mechanism
- Automatic row by row single side push-in/pull-out loading/unloading
- Integrated Steps
- Lyophilization, Solvent Handling
- CIP/SIP
- CIP and SIP Included
- Automation Level
- SCADA Controlled
- Isolation Type
- Mushroom type chamber-condenser isolation valve
- Vacuum System
- Dry Vacuum Pumps
- Integration
- Integration with Isolator
- Density/particle size
- 0.5–2.5 g/cm³ / 50–1000 µm
- Machine footprint
- 1 X 20 m² freeze dryer
- Ice condenser capacity
- 400 kg
- Chamber-condenser isolation valve
- Mushroom type
- Loading/unloading system
- Automatic row by row single side push-in/pull-out
- Integration
- With isolator
- Control package
- SCADA
- Control panel type
- SCADA control package
- Integration possibilities
- Integration with isolator
- Loading/unloading system
- Automatic row by row single side push-in/pull-out
- Chamber-condenser isolation valve
- Mushroom type