Modular pharmaceutical formulation systems
Achieve unparalleled flexibility and efficiency in liquid pharmaceutical formulations by integrating modular systems designed to streamline mixing, filling, and purification processes while ensuring compliance with strict regulatory standards.
Processes and Sterilizes Liquid Parenteral Pharmaceuticals
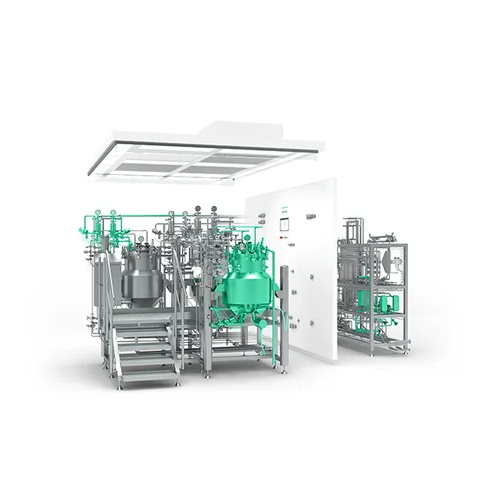
The SVP Essential and LVP systems from Syntegon provide modular solutions tailored for liquid pharmaceutical manufacturing. Designed for flexibility, these systems handle mixing, homogenizing, and filling of both small and large volume parenterals. With capacities ranging from 13 to 20,000 liters, they integrate seamlessly into production lines through automation, enabling efficient batch and continuous operations. These systems feature robust CIP/SIP capabilities, ensuring rapid, contamination-free cleaning. Constructed with stainless steel, they comply with GMP and FDA standards, making them ideal for antibiotics, infusion solutions, and cell culture media production. The prefabricated cleanroom design reduces installation time and guarantees a minimal footprint, adapting to various production requirements. With over 70 pre-tested modules and ISA-88 structured automation, Syntegon’s solutions ensure process precision and operational control.
Benefits
- Enhances flexibility in production with scalable batch sizes from 13 to 20,000 liters.
- Streamlines operations through integrated CIP/SIP systems for efficient cleaning.
- Ensures compliance and quality with GMP and FDA certification.
- Adapts easily to existing facilities with modular, prefabricated cleanroom designs.
- Increases operational efficiency with ISA-88 automation architecture for precise control.
- Applications
- Buffer preparations, Infusion products, Media preparations, Small volume parenterals, Large volume parenterals, Pharmaceuticals
- End products
- Dextrose solutions, Pharmaceutical buffer solutions, Total parenteral nutrition (tpn) solutions, Cell culture media, Antibiotics, Amino acid solutions, Small volume injectable drugs, Saline solutions, Anticoagulants, Intravenous hydration solutions, Electrolyte solutions, Microbial culture media
- Steps before
- Purification, Formulation, Mixing, Substance addition, Homogenization, Sterile filtration
- Steps after
- Sterilization, Filling, Capping, Inspection, Packaging
- Input ingredients
- small volume parenterals (SVP), large volume parenterals (LVP), infusion products (IV), buffer preparations, media preparations, APIs, excipients, sterile substances, toxic substances, aqueous solution, suspension parenteral products
- Output ingredients
- liquid pharmaceuticals, parenteral drug products, infusion products, buffer preparations, media preparations, final product packaging, small volume parenterals (SVP), large volume parenterals (LVP)
- Market info
- Syntegon is known for its expertise in processing and packaging technology, offering innovative solutions for the food and pharmaceutical industries. It has a strong reputation for advanced equipment, sustainability, and customer-oriented service.
- Batch size
- 13-20, 000 liters
- Automation
- Fully integrated and automated
- Mixing options
- Low and high shear mixers, high-performance homogenizers and emulsifiers, vibration mixers
- Sterile filtration
- Integrated sterile filter stations
- Room classification
- Different room classifications
- Functional modules
- Over 70 pre-tested functional modules
- CIP/SIP system
- Shared CIP/SIP interface
- Process vessel type
- Modular temperature-controlled stainless-steel tanks
- Cooling options
- Temperature-controlled
- Manual and automated feeding
- APIs and excipients
- Product types
- Solutions, suspensions, emulsions
- Cleanroom design
- Prefabricated integrated design
- Process control
- ISA-88 structured automation architecture
- Batch vs. continuous operation
- Batch
- CIP/SIP
- CIP 121°C / SIP 135°C
- Automation level
- PLC / SCADA
- Energy efficiency
- 0.5–2 kWh/kg moisture
- Cleaning method
- CIP / Manual
- Changeover time
- 0
- Corrosive resistance (e.g. acids)
- Yes
- Density/particle size
- 0.5–2.5 g/cm³ / 50–1000 µm
- Tank shape
- Stainless-steel tanks
- Modular building set
- Pre-tested functional modules
- Room classification flexibility
- Different room classifications available
- Control Panel Type
- HMI or PLC
- Integration Possibilities
- Modular Infrastructure Integration
- Room Layout Flexibility
- Customizable Cleanroom Design
- Modular Building Sets
- Over 70 Pre-tested Modules
- Interface to Filling Line
- Optional Shared CIP/SIP
- Container Configuration
- Fixed and Mobile
- Substance Addition Options
- Manual or Automated
- Automation System
- ISA-88 Structured
- Process Vessel Customization
- Shape and Volume Selection
- Functional Module Configuration
- Joint Selection With Customer
- Recipe Implementation
- Program, Production, Batch Management