Aseptic syringe filling solution for Rtu containers
Streamline your pharmaceutical liquid filling operations with precise aseptic syringe filling to ensure high-quality sterile production, minimize contamination risks, and enhance product consistency across varying batch sizes.
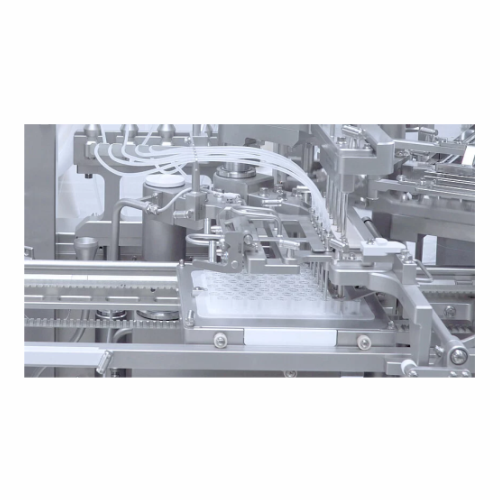
Fills and Assembles Aseptic RTU Containers
The flexfill from groninger is an advanced filling machine designed for precise handling of aseptic pharmaceutical products in ready-to-use (RTU) containers. It specializes in filling and closing pre-sterilized syringes, vials, and cartridges, making it ideal for pharmaceuticals such as pre-filled syringes and vial-based vaccines. Employing no-touch transfer (NTT) and E-Beam decontamination options, it ensures contamination-free transfer into aseptic environments. Handling capacities range from small pilot batches to high-speed industrial production, accommodating up to 1,000 containers per minute, all controlled via a PLC system for seamless integration into production lines. Meeting strict GMP compliance, flexfill optimizes throughput with features like smartfill technology for minimal product loss and robust energy efficiency. Stainless steel construction ensures chemical compatibility, and automated in-process control (IPC) assures precise filling accuracy, making it a reliable choice for complex biopharmaceutical manufacturing needs.
Benefits
- Optimizes production efficiency with rapid changeovers and minimal downtime.
- Ensures precise dosage accuracy, reducing waste and enhancing product reliability.
- Minimizes contamination risk with advanced decontamination and no-touch transfer technologies.
- Adapts to varying batch sizes, offering flexibility for both small-scale and large-scale production.
- Complies with GMP standards, ensuring high-quality pharmaceutical manufacturing.
- Applications
- Pharmaceutical solutions, Liquid pharmaceuticals, Aseptic pharmaceutical products, Biopharmaceuticals, Ready-to-use packaging, Pharmaceuticals in rtu containers
- End products
- Vial-based vaccines, Cartridge-packed insulin, Ophthalmic solution dropper vials, Hormonal injection vials, Nest-packed biologics, Pre-filled syringes, Respirable nasal sprays, Single-use vaccine doses, Liquid antibody pharmaceuticals, Injectable synthetic hormones
- Steps before
- Container infeed, Outside decontamination, Aseptic no-touch transfer (NTT)
- Steps after
- Inspection, Labeling, Plunger rod installation, Finger flange assembly, Safety device assembly, Final packaging
- Input ingredients
- ready-to-use vials, ready-to-use syringes, ready-to-use cartridges, pre-sterilized glass objects, pre-sterilized polymer objects, nested RTU containers, single or double bags, aseptic no-touch transfer (NTT), H2O2 decontamination, E-Beam decontamination
- Output ingredients
- pre-filled syringes, filled vials, filled cartridges, sealed vials with aluminum caps, labeled RTU containers, assembled syringes, assembled finger flange, assembled safety devices, downstream processed packaging
- Market info
- Groninger is known for its expertise in manufacturing high-quality filling and packaging machinery for the pharmaceutical, cosmetics, and consumer healthcare industries, with a strong reputation for innovation, precision engineering, and customer-focused solutions.
- Compliance
- GMP compliant
- Container Type
- Pre-sterilized glass and polymer
- Filling Automation
- Automated syringe fillers
- Transfer Technology
- No-touch transfer (NTT), E-Beam, H2O2
- Opening Technology
- Patented pull-off rollers
- Filling Control
- Statistical IPC, 100% IPC
- Processing Speed
- Up to 60, 000 objects per hour
- Batch Sizes
- Small batches to large-scale production
- Closure Method
- Single-disk flanging principle
- Freeze Dryer Integration
- Manual to automatic up to 600/min
- Standard Gassing and Vacuum Functions
- Included
- Syringe Filling Systems
- Rotary piston pumps, peristaltic pumps, time-pressure system
- Cleanroom Compatibility
- Class A, ISO 5
- Smartfill Feature
- Minimizes product loss
- No-touch transfer (NTT)
- Basic / Advanced
- Automation level
- Manual / Semi-automatic / Fully automatic
- Changeover time
- Minimal without tools
- Process zone transfer
- Grade A aseptic process
- Integrated decontamination
- H2O2 / E-Beam
- Process flexibility
- Small-batch to high-volume
- Product loss minimization
- Smartfill feature
- Fill weight control
- Statistical IPC / 100% IPC
- Abrasion resistance
- High
- Biological compatibility
- Suitable for pharmaceutical applications
- Cleanability
- Easy to clean, supports GMP standards
- Corrosive resistance (e.g. acids)
- Good, designed for various disinfectants
- Density/particle size
- Adaptable for various RTU containers
- Nesting configuration
- Tub / Nest
- GMP Compliance
- Yes
- FDA Materials
- Yes
- ISO 14644
- Class 5
- Annex 1 Compliance
- Yes
- CFR 21 Part 11
- Yes
- Compact footprint
- Yes, designed for cleanroom integration
- Discharge method
- Semi-automatic and fully automatic
- Control panel type
- Touchscreen interface
- Machine footprint
- Optimized for pharmaceutical cleanrooms
- Configuration
- Modular for easy changeover
- Control panel type
- HMI / Touchscreen
- Integration possibilities
- SCADA / MES
- Footprint
- Compact / Modular
- Customizable filling systems
- Rotary piston / Peristaltic / Time-pressure
- Flexible batch sizes
- Small / Medium / Large
- Tool-free format changeover
- Yes
- Modular design
- Yes
- Automation level
- Manual / Semi-automatic / Fully Automatic