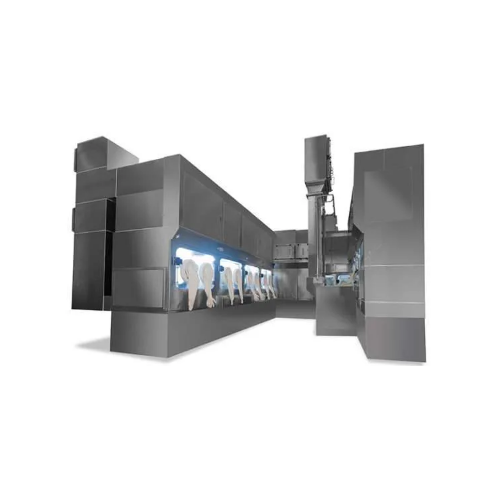
Freeze dryer & filling line aseptic isolator for cytotoxic products
Handle cytotoxic and high-potent drugs with precision and safety using this engineered freeze dryer and isolator, ensuring aseptic conditions and reliable lyophilization in demanding pharmaceutical processes.
Processes Cytotoxic and Hygroscopic Pharmaceuticals Safely
The Freeze Dryer & Aseptic Isolator Filling Line from Telstar is engineered to meet stringent pharmaceutical and biotech production needs. This integrated system performs critical tasks such as filling, lyophilization, capping, and crimping of cancer treatment vials and cytotoxic injectables while ensuring aseptic conditions. It employs a sterile multi-chamber configuration with VHP biodecontamination and advanced monitoring systems to maintain operator safety and product integrity. Designed for high potency and hygroscopic products, this equipment handles both batch and continuous operations with precision. It features cold shelf loading and low-temperature air handling units for effective lyophilization, ensuring the stability of delicate compounds. The inclusion of RFID glove testers and advanced isolation units supports seamless integration into existing lines, while compliant with GMP standards. The use of stainless steel construction enhances durability and corrosion resistance, reducing maintenance burdens and ensuring longevity.
Benefits
- Ensures operator safety by maintaining exposure levels below 1µg/m³.
- Enhances product purity through precise aseptic handling and lyophilization.
- Integrates seamlessly into existing lines with advanced monitoring and control systems.
- Minimizes contamination risks with VHP biodecontamination and RFID glove testing.
- Compliant with GMP standards, ensuring regulatory adherence and product quality.
- Applications
- High potent drugs, Cytotoxic products, Innovative drugs, Biopharmaceuticals, Anticancer drugs, Hygroscopic products
- End products
- Anti-infective lyophilized powders, Lyophilized peptides, Cancer treatment vials, Monoclonal antibodies, Cytotoxic injectables, Chemotherapy injectable solutions, Antibody-drug conjugates (adcs), High-potency active pharmaceutical ingredients (hpapis), Lyophilized oncological drugs, Small molecule apis
- Steps before
- Purification, Aseptic liquid filling, Sterile multi-chamber filling
- Steps after
- Lyophilisation, Capping, Crimping, Washing, Sterile lyophilised filling
- Input ingredients
- aseptic liquid, vials, cytotoxic products, hygroscopic products
- Output ingredients
- lyophilized vials, capped vials, crimped vials, washed vials
- Market info
- Telstar is known for specializing in the design, engineering, and manufacturing of advanced vacuum and aerospace solutions, gaining a strong reputation for their innovative and customized equipment in pharmaceutical, medical, and scientific research sectors.
- Automation
- Integrated VHP biodecontamination system
- Occupational Exposure Limit (OEL)
- Less than 1 µg/m³ TWA over 8hr
- System Type
- Sterile Multi-Chamber Filling/Lyophilisation Line
- Number of Modules
- 7 module system
- Temperature Handling
- Low temperature air handling unit
- Lyophilizer Door Type
- Front slot door, side gimbal type maintenance door
- Automation level
- Manual / PLC / SCADA
- Batch vs. continuous operation
- Batch
- CIP/SIP capability
- CIP 121°C / SIP 135°C
- Occupational Exposure Limit (OEL)
- <1µg/m³ TWA over an 8hr operational period
- Density/particle size
- 0.5–2.5 g/cm³ / 50–1000 µm
- Isolator system type
- Multi-Chamber
- Number of modules
- 7
- Shelf configuration
- Cold shelf loading
- Condenser type
- Vertical condenser
- Maintenance door type
- Side gimbal type
- Control Panel Type
- Touchscreen HMI
- Integration Capabilities
- VHP Biodecontamination System Integration
- RFID Glove Tester Compatibility
- Yes
- Cold Shelf Loading System
- Available
- Monitoring System Type
- Viable, Non-viable Monitoring
- Conveyor Type
- Buffer Conveyors
- Low Temperature Air Handling Unit
- Available