Closure system for aseptic containment
Achieve secure and verifiable seals for sterile pharmaceuticals with advanced closure systems, ensuring contamination-free handling and maintaining product integrity in critical environments.
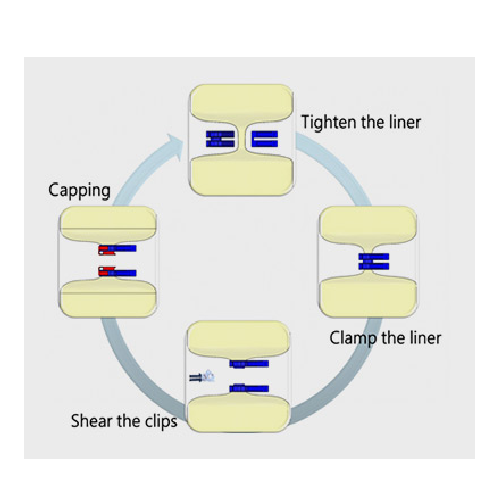
Ensures Secure Sealing and Tamper-Proofing
The Closure System from AUSTAR provides pharmaceutical manufacturers and biotechnology companies with a robust solution for aseptic sealing and tamper-proofing. Utilizing high-strength PA materials with a high melting point, the system ensures reliable and verifiable sealing even under challenging conditions. It’s engineered to minimize dust exposure during the cutting process, making it particularly suitable for the packaging of sterile injectable medications and parenteral nutrition solutions. The system supports various processes such as crimping, cutting, and bag opening through specialized tools like crimping pliers and cutting tools. It seamlessly integrates with manual and high-speed operations, handling both solid and powder product types. The inclusion of tamper-proof sealing labels enhances security, providing an additional layer of integrity for your products. Designed to meet industry standards in pharmaceutical, biotech, and laboratory settings, the Closure System ensures compliance with GMP and FDA regulations.
Benefits
- Ensures product integrity with tamper-proof sealing, enhancing security in packaging.
- Minimizes contamination risk through reliable and verifiable sealing processes.
- Supports seamless integration into existing production lines with customizable toolsets.
- Meets stringent industry standards, ensuring compliance with GMP and FDA regulations.
- Reduces maintenance time with easy-to-use clamping and shearing tools designed for stable performance.
- Applications
- Aseptic containment products, Biopharmaceuticals, Capsules, Pharmaceutical tablets
- End products
- Gelatin capsules, Single-use bioreactor bags, Pre-filled syringes, Blister packs of tablets, Parenteral nutrition solutions, Vials of sterile injectable medication, Sterile iv bags
- Steps before
- Packaging, Loading into continuous liner, Transfer preparation
- Steps after
- Tamper-proof labeling, Final sealing verification, Quality inspection
- Input ingredients
- continuous liner, package of products, BIBO
- Output ingredients
- sealed packages, secure transfers
- Market info
- Austar is known for specializing in the design and manufacture of engineered-to-order industrial equipment, particularly in the pharmaceutical and biotechnology sectors, offering solutions that focus on quality, innovation, and meeting specific customer requirements.
- Material
- High strength PA material
- Melting point
- High
- Solubility
- Insoluble in common solvents
- Locking Mechanism
- Double locking
- Sealing Performance
- Stable and verifiable
- Dust Exposure
- Extremely low
- Tamper-proof
- Yes, destructive opening
- Tools
- Crimping Pliers, cutting tools, bag-opener, sealing label
- Sealing Method
- Continuous liner / BIBO
- Locking Mechanism
- Double locking, safe and reliable
- Sealing Stability
- Stable and verifiable
- Cutting Performance
- Tight and flat section, low dust exposure
- Tamper-Proof Feature
- Destructive opening labels
- Material Performance
- High strength PA, high melting point, solvent insoluble
- Corrosive resistance (e.g. acids)
- High strength PA material, insoluble in common solvents
- Destructive opening
- Tamper-proof
- Material
- High strength PA
- Melting Point
- High
- Locking Mechanism
- Double locking
- Sealing Performance
- Stable and verifiable
- Cutting Section Finish
- Tight and flat
- Locking mechanism
- Double locking