Bioprocess system for protein and antibody drugs
Streamline biopharmaceutical production with a system engineered to optimize the upstream and downstream processing of recombinant proteins, vaccines, and monoclonal antibodies, enhancing efficiency and product yield.
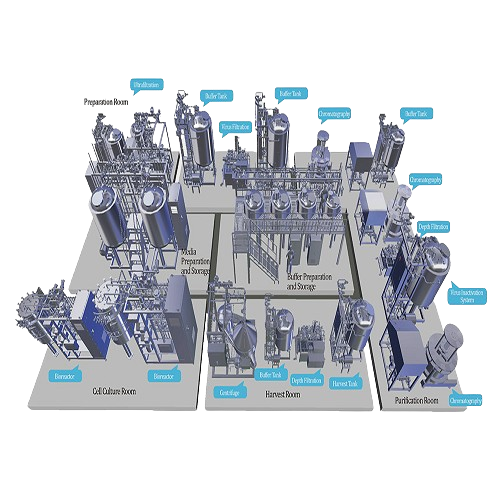
Integrates Upstream and Downstream Bioprocess Operations
The Bioprocess System from AUSTAR is engineered to support the demanding needs of biopharmaceutical companies and research institutions. This system excels in integrating upstream and downstream operations, employing cutting-edge stainless steel and single-use technologies to handle processes such as fermentation, chromatography, and virus removal efficiently. Designed to produce monoclonal antibodies, vaccines, and gene therapy vectors, it facilitates scalable production from pilot to commercial levels. The system can process various forms, maintaining product integrity while ensuring compliance with ISO9001 and ASME-BPE standards. With automation capabilities that include PLC controls and remote monitoring, it ensures seamless integration into production lines. The equipment’s energy efficiency is optimized through advanced motor controls, aligning with stringent sustainability objectives. It is available with customizable options for materials and configurations, backed by comprehensive engineering support for tailored production solutions.
Benefits
- Enhances production efficiency with fully integrated upstream and downstream processes.
- Reduces operational costs by optimizing energy consumption through variable-speed motor technology.
- Ensures product quality and safety with compliance to ISO9001 and ASME-BPE standards.
- Minimizes contamination risk with customizable, single-use system options.
- Facilitates scale-up from pilot to commercial production, supporting flexible production goals.
- Applications
- Vaccines, Antibody drugs, Biopharmaceuticals, Blood products, Recombinant protein drugs
- End products
- Erythropoietin, Intravenous immunoglobulin (ivig), Monoclonal antibodies, Covid-19 vaccines, Coagulation factor concentrates, Gene therapy vectors, Insulin analogs, Human albumin, Influenza vaccines, Hepatitis b vaccines
- Steps before
- Media Preparation, Buffer Preparation, Fermentation, Bacteria Crushing
- Steps after
- Clarification, Harvest, Dispensing, Filling
- Input ingredients
- recombinant protein drugs, antibody drugs, vaccines, blood products, media preparation, buffer preparation
- Output ingredients
- core process equipment, biopharmaceutical upstream process equipment, biopharmaceutical downstream process equipment, fermentation system, bioreactor, chromatographic system, product clarification, harvest, deep filtration process, ultrafiltration process, centrifugal process, concentrate dispensing process
- Market info
- Austar is known for specializing in the design and manufacture of engineered-to-order industrial equipment, particularly in the pharmaceutical and biotechnology sectors, offering solutions that focus on quality, innovation, and meeting specific customer requirements.
- System Type
- Upstream/Downstream Core Bioprocess
- Material
- Stainless Steel / Single-use Systems
- Compliance Standards
- ISO9001, ASME-BPE
- Automation
- Customizable
- Process Module Types
- Deep Filtration, Di-virus, Ultrafiltration, Centrifugal
- Application Range
- Research and Development to Commercial Scale Production
- Customization
- Integrated Engineering Solutions
- Fermentation System
- Bioreactor Equipped
- Filling Solution
- Preparation Dispensing, Filling
- Product Types
- Recombinant Protein Drugs, Antibody Drugs, Vaccines, Blood Products
- Filtration System
- Deep Filtration Process Module
- Dispensing Process Solution
- Concentrate Dispensing
- Working mechanism
- Fermentation/bioreactor, chrom-atographic separation
- Process integration
- Upstream/Downstream core process
- Cleaning method
- CIP/SIP
- Automation level
- Manual/PLC-controlled
- Batch vs. continuous operation
- Batch/Inline operation
- Density/particle size
- 0.5–2.5 g/cm³ / 50–1000 µm
- Compact footprint
- Yes
- Customization options
- Stainless steel system / Single-use system
- Control panel type
- PLC/SCADA
- Integration possibilities
- Upstream/Downstream systems
- System design
- Stainless steel/Single-use
- Process modules
- Fermentation/Bioreactor/Chromatographic
- Cleaning options
- CIP/SIP
- Engineering solutions
- Media preparation/Distribution