Biowaste decontamination system
Ensure safe handling of pathogenic and genetically modified organisms in wastewater with precise decontamination and inactivation processes tailored to meet stringent safety standards.
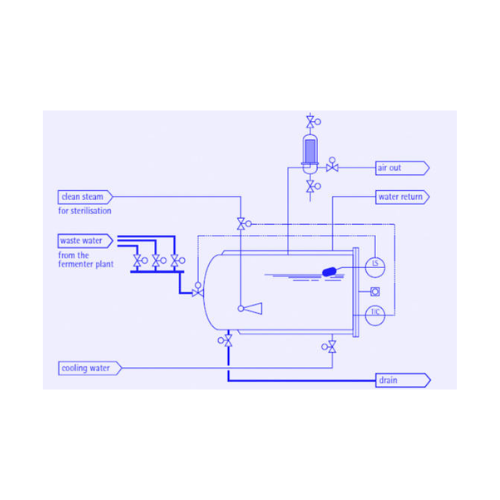
Deactivates and Sterilizes Contaminated Wastewater
The Killtank Wastewater Decontamination System, offered by Bioengineering AG, is designed to address the critical production need for the deactivation of pathogenic and genetically modified organisms in wastewater. This system employs a combination of batch or continuous heat sterilization processes to ensure thorough decontamination, adhering to local regulatory standards.
Ideal for biopharmaceutical manufacturers, research labs, and wastewater treatment facilities, the Killtank system plays a vital role in producing sterile effluents and safeguarding the environment. It features automated controls for both batch and continuous modes, integrating seamlessly into existing processes with options for manual operation where needed.
The system incorporates heat exchangers capable of pre-heating wastewater to 100°C and then elevating temperatures to 140°C for complete inactivation, followed by rapid cooling. Additionally, energy efficiency is enhanced through a built-in heat recovery system.
Constructed from materials that offer superior corrosion resistance, the Killtank is compliant with stringent safety and environmental standards, making it a reliable choice for managing biohazardous waste. Maintenance is simplified with an integrated Cleaning In Place (CIP) system, facilitating quick, contamination-free cleaning and ensuring minimal downtime.
Benefits
- Ensures environmental compliance with reliable deactivation of hazardous organisms.
- Lowers operational costs through integrated energy-efficient heat recovery.
- Facilitates seamless integration with automated controls, reducing manual oversight.
- Provides versatile operation options for both batch and continuous processes.
- Offers robust corrosion resistance for increased equipment longevity.
- Applications
- Biopharmaceuticals, Genetically modified organisms, Wastewater treatment, Pathogenic organisms
- End products
- Sterile effluents, Vaccines, Insulin, Genetically modified bacteria deactivation, Monoclonal antibodies, Pathogen-free wastewater, Recombinant proteins
- Steps before
- Fermentation, Collection of contaminated wastewater
- Steps after
- Heat sterilization, Cooling, Discharge into sewer system
- Input ingredients
- contaminated wastewater, pathogenic organisms, genetically modified organisms
- Output ingredients
- deactivated wastewater, inactivated wastewater, sterilized wastewater
- Market info
- Bioengineering is known for its expertise in designing and manufacturing customized bioreactors and fermenters, serving the biotechnology and pharmaceutical industries. They are reputed for high-quality, engineered-to-order solutions and innovative process technologies.
- Decontamination Method
- Heat inactivation
- Automation
- Full automation possible
- Operation Mode
- Batch / Continuous
- Temperature
- 100°C – 140°C
- Process Documentation
- Fully documented
- Heat Recovery System
- Integrated
- Pre-heating Temperature
- 100°C
- Main Heating Temperature
- 140°C for 30 seconds
- Cooling Process
- Post-sterilization cooling
- Working mechanism
- Batchwise or continuous decontamination
- Integrated steps
- Collection, heat sterilization, cooling, discharge
- CIP/SIP
- Heat inactivation
- Batch vs. continuous operation
- Batch / Continuous
- Automation level
- Manual / Full automation
- Cleaning method
- Steam injector, heat sterilization
- Decontamination Purpose
- Pathogenic, Genetically Modified Organisms
- Tank shape
- Cylindrical
- Tank size
- Varies by model/configuration
- Footprint
- Compact design for lab and pilot scale
- Discharge method
- Steam injector for heat-sterilization




