Aseptic transfer port for secure bi-directional transfer
Ensure seamless and contamination-free transfer of sterile materials, optimizing the safety and efficiency of your pharmaceutical production process with cutting-edge containment technology.
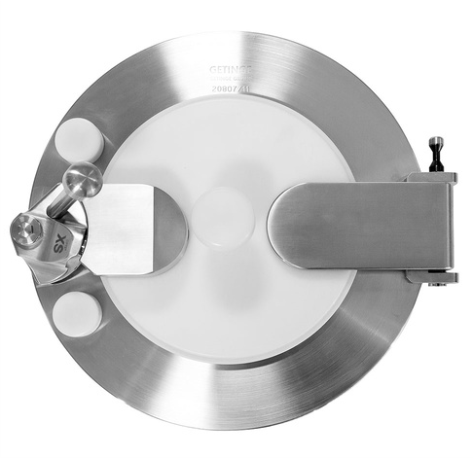
Ensures Sterile and Contained Transfers
The DPTE®-XS Alpha Port by Getinge offers a robust solution for secure, sterile transfer in challenging environments. Designed to maintain aseptic conditions, this innovative system connects cleanroom or isolator walls to Beta devices like containers or bags, ensuring a leak-tight and contamination-free transfer. With industry applications spanning pharmaceutical production, biotech operations, and chemical sectors, it’s essential for processes like liquid and component transfer, environmental monitoring, and waste disposal. Its integration into existing systems is seamless, minimizing downtime and operational interruption.
A validated DPTE® system features reliable Alpha/Beta connections, supported by FDA-compliant silicone or PVC lip seals, ensuring each transfer is performed with the utmost safety and efficiency. The manual 60° rotation mechanism guarantees a leak-tight seal and secure connections, while its maintenance-free design and sterile, ready-to-use components reduce downtime and maximize production uptime. Designed to comply with stringent international regulations, the DPTE®-XS Port is a trusted choice for any sterile or containment process requiring rigorous quality assurance and reliable expert support.
Benefits
- Ensures sterility and containment, reducing contamination risk in critical processes.
- Enhances operational efficiency with easy and leak-tight manual connection.
- Complies with international safety standards, supporting rigorous regulatory requirements.
- Minimizes downtime through ready-to-use, maintenance-free components.
- Integrates seamlessly into existing production lines, optimizing workflow without disruption.
- Applications
- Aseptic production, Component transfer, Liquid transfer, Machine parts, Contained production, Biomedical components, Waste disposal, Environmental monitoring, Pharmaceutical production
- End products
- Sterile injectable drugs, Biologics, Implantable medical devices, Cell therapy products, Sterile pharmaceutical containers, Vaccines, Sterile iv fluids, Hazardous drug compounds, Contaminated laboratory waste, Diagnostic reagents
- Steps before
- Component Preparation, Liquid Preparation, Material Loading
- Steps after
- Sterilization, Containment, Environmental Monitoring, Waste Disposal
- Input ingredients
- components, solids, liquids
- Output ingredients
- aseptic transfer, sterile transfer, contained transfer
- Market info
- Getinge is known for providing innovative healthcare and life sciences products, specializing in medical technology, infection control, surgical workflows, and critical care solutions. They maintain a strong reputation for quality and technological advancement in these sectors.
- Diameter
- 105 mm / 190 mm / 270 mm / 350 mm / 460 mm
- Useful Diameter
- 95 mm / 170 mm / 250 mm / 330 mm / 440 mm
- Available Lip Seal
- FDA Compliant Silicone / PVC
- Leak Tightness
- Lip seals ensure leak-tight seal upon connection
- Connection Method
- Manual 60° rotation for secure lock
- Automation
- Manual operation with manual rotation
- Batch vs. continuous operation
- Batch / Inline Continuous
- Cleaning method
- CIP / Manual
- Automation level
- Manual / PLC / SCADA
- Energy efficiency
- 0.5–2 kWh/kg moisture
- Density/particle size
- 0.5–2.5 g/cm³ / 50–1000 µm
- Compatibility with Beta parts
- Yes
- Installation environment
- Cleanroom / Isolator / RABS / BSC
- FDA Materials
- FDA Compliant Silicone
- Port Diameter
- 105 mm, 190 mm, 270 mm, 350 mm, 460 mm
- Useful Diameter
- 95 mm, 170 mm, 250 mm, 330 mm, 440 mm
- Seal Type
- FDA Compliant Silicone, PVC
- Integration possibilities
- Isolators, RABS, BSC, cleanroom
- Port diameter
- 105 mm, 190 mm, 270 mm, 350 mm, 460 mm
- Lip seal material
- FDA Compliant Silicone, PVC
- Beta part compatibility
- Containers, bags, trolleys