Serial Data Managing Database
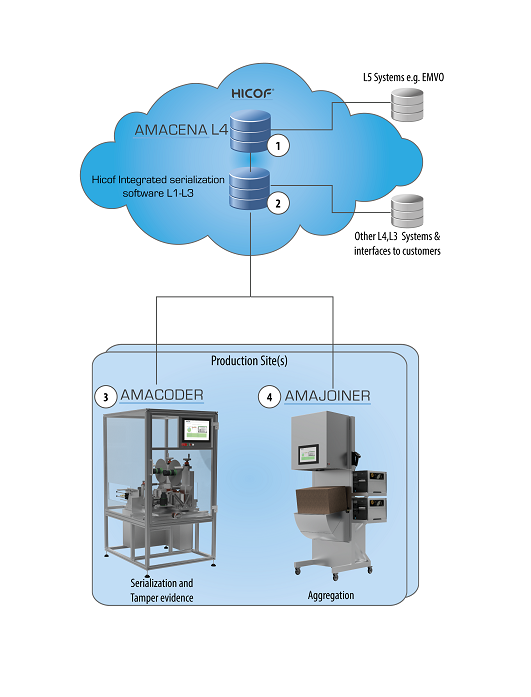
Managing serialization data is a vital part of accurate reporting and product integrity in the pharmaceutical industry. Current systems require a cascade of servers from line level to site level and up to corporate level. Usually supplied by different vendors, interfaces are required between all these systems which can introduce integrity issues as well as adding complexity. An all-in-one solution can overcome these challenges and provide a “single source of truth” for all data.
Cost-effective cloud solution for whole-organisation integrated serialization management
The Amacena System from Hicof is a completely integrated single system for managing serialization data across your entire business.
The system is a cloud-based subscription model which offers substantial cost benefits as well as redundancy. There is also an on-premises client-server option where this may be preferred.
Key benefits to pharmaceutical producers include the data integrity and streamlining benefits of operating a single vendor system from levels 1 to 4 across the business, ensuring data integrity at all stages.
Amacena allows fully GMP compliant Master Data Management, providing a single source of truth for data across the organization.
Cloud technology allows full integration with external partners on a global scale, including regulators, contract manufacturing organizations and marketing authorization holders.
By offering three installation and operation options, the system can be completely tailored to your exact requirements – full cloud, hybrid, full on-premises.
Benefits
- Private cloud solution reduces data management costs
- Single source of truth for data across the entire organization
- Easy to operate, no external expertise required
- Full external integration, including governmental, CMO, MAH
- Free software updates for cloud customers