Bench-top syringe & cartridge closing system
Ensure precise and repeatable syringe and cartridge closure with this versatile bench-top system, designed for small-batch operations in pharmaceutical environments, offering flexibility with vacuum and vent-tube insertion methods.
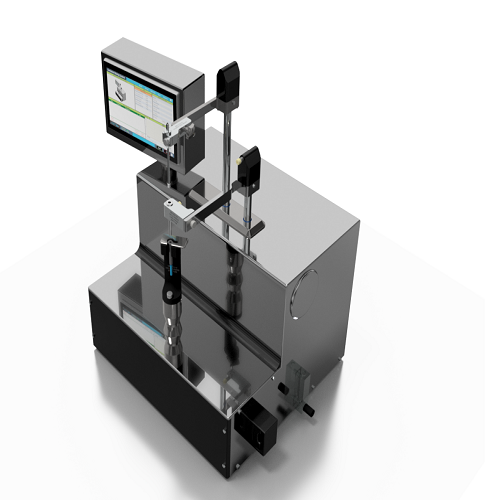
Closes Syringes and Cartridges Precisely
The Container Closing System (CCS) from AST is a benchtop, semi-automatic machine designed to provide precise control over the syringe and cartridge closing process. This system stands out for its use of both vacuum and vent-tube methods, accommodating a range of container sizes with minimal format changes. Ideal for biologics, vaccines, and personalized medicine, the CCS ensures contamination-free sealings, such as those required for monoclonal antibodies and mRNA vaccines.
Operating under aseptic conditions, the system supports small-batch production with its compact footprint, making it suitable for laboratory and cleanroom environments. Key applications include clinical trial materials manufacturing, compounding pharmacies, and processing cell and gene therapies. The electronic control system offers intuitive operation via an HMI touchscreen, allowing operators to create and monitor precise insertion recipes.
Featuring AST’s patented multi-stage vacuum insertion process, the CCS guarantees consistent piston placement, essential for biologic and compounded pharmaceutical products sensitive to oxidation. An optional Electronic Batch Reporting (EBR) system enhances batch documentation, regulatory compliance, and technology transfer. Constructed from pharmaceutical-grade stainless steel, the system also offers compatibility upgrades for isolator use, ensuring compliance with GMP standards.
Benefits
- Ensures precise and repeatable syringe and cartridge sealing, critical for product integrity in biomedical applications.
- Reduces operator intervention and potential contamination with semi-automatic operation and easy-to-change format parts.
- Enhances safety and compliance with aseptic design suitable for cleanroom and isolator environments.
- Facilitates compliance and process documentation with optional Electronic Batch Reporting (EBR) system.
- Adapts to various production needs with flexible vacuum and vent-tube insertion processes.
- Applications
- Biologics, Compounded pharmaceuticals, Personalized medicine, Vaccines, Bio-similars, Cell and gene therapy products
- End products
- Recombinant erythropoietin, Car-t cell therapies, Monoclonal antibodies, Mrna vaccines, Compounded pain relief syringes, Personalized cancer vaccines
- Steps before
- Manual placement of filled syringe or cartridge, Pre-fill container purging with inert gas
- Steps after
- Batch documentation, Process analysis, Technology transfer, Regulatory filing, Placement within an isolator, bio-safety cabinet or laminar airflow hood
- Input ingredients
- pre-filled syringes, cartridges, air, mechanical assist, vent-tube, vacuum
- Output ingredients
- sealed syringes, sealed cartridges, closed containers, inserted piston, evacuated air
- Market info
- AST is known for its expertise in designing and manufacturing customized engineered-to-order equipment for industrial applications, renowned for precision engineering, innovation, and reliable solutions tailored to meet unique customer specifications and industry standards.
- System Dimensions
- 780mm x 500mm x 740mm [31" x 20" x 29"]
- Container Types
- Syringes, Cartridges
- Syringe Sizes
- 0.5mL - 50mL
- Cartridge Sizes
- 1mL to 20mL
- Available Closing Options
- Vacuum, Vent-Tube, , Vent/Vac Combo
- HMI
- ASTView on a 10" color touchscreen
- Materials of Construction
- Pharmaceutical grade stainless steel, plastics, and elastomers
- Automation
- Electronically controlled process
- Batch documentation
- Electronic Batch Report (EBR) System
- Process Flexibility
- Vacuum or Vent-Tube insertion
- Insertions
- Mechanical vent-tube and vacuum piston insertion
- Compact Design
- Suited for aseptic isolators, biological safety cabinets or laminar airflow hood
- Working mechanism
- Semi-automatic
- Cleanroom compatibility
- ISO compliant
- Compact design
- Yes
- Automation level
- Electronic control
- Process flexibility
- Vacuum and Vent-Tube
- Tool-less format change
- Yes
- Batch documentation
- Optional EBR system
- Changeover time
- Quick with format part changes
- Cleaning method
- cGMP designed
- Biological compatibility
- Aseptic design suitable for biologics
- Cleanability
- Compatible with cleanrooms, bio-safety cabinets
- Corrosive resistance (e.g. acids)
- Pharmaceutical grade stainless steel
- Aseptic design
- Compatible with isolators, safety cabinets
- System Dimensions
- 780mm x 500mm x 740mm [31" x 20" x 29"]
- Compact design
- Aseptic isolators, biological safety cabinets, laminar airflow hood compatibility
- Control panel type
- ASTView high-resolution interface on a 10" color touchscreen
- Control panel type
- HMI ASTView on a 10" color touchscreen
- Integration possibilities
- Compatibility with isolators, bio-safety cabinets, laminar airflow hoods
- Process flexibility
- Vacuum or Vent-tube insertion processes with format part changes
- Electronic Batch Report (EBR) System
- Optional feature for batch documentation
- System footprint
- Compact design